Summer 2022
Welcome from EUS-AAEM
The Emergency Ultrasound Section of the American Academy of Emergency Medicine (EUS-AAEM) is founded to foster the professional development of its members and to educate them regarding point-of-care ultrasound. This group will serve as a venue for collaboration among medical students, residents and practitioners who are interested in point-of-care ultrasound. The purpose of our group is to augment the knowledge and expertise of all emergency medicine specialists and to advocate for patient safety and quality care by endorsing bedside ultrasound. Membership is not limited to fellowship trained physicians. All emergency medicine practitioners passionate about ultrasound are welcome to join and participate.
We are proud to publish our e-newsletter with original contributions from many of our members. We encourage all members to submit for future editions. Topics include but are not limited to educational, community focus, interesting cases, resident and student section, and adventures abroad.
For more information visit us online at: www.aaem.org/EUS.
In this Issue:
-
Chair's Message
-
Ultrasound Building Blocks: Point-of-Care Transorbital Ultrasound for the Diagnosis of Optic Neuritis: A Case Report
-
Resident Section: Hidden in Plain Sight: Impending Paradoxical Embolism
-
Fellow Section: Use of Resuscitative Transesophageal Echocardiography in the Emergency Department to Diagnose Pulmonary Embolism
-
Ultrasound Guided Procedures: Ultrasound Guided Ganglion Cyst Aspiration from the Dorsum of the Wrist
-
Ultrasound Saves: TampoNOT: Use of POCUS to differentiate a mistaken case of acute cardiac tamponade
-
Ultrasound Saves: POCUS for Ocular Trauma
-
Glimpse of Original Research: Creating a Novel 3D-Printed Phantom to Simulate Ultrasound-Guided Pericardiocentesis
-
Glimpse of Original Research: Point of Care Ultrasound Diagnosis of Deep Vein Thrombosis by Medical Students
-
Glimpse of Original Research: Utilizing 3-dimensional cardiac models with point of care ultrasound video tutorials to improve anatomic identification during medical student first time ultrasound exposure, a double-blinded randomized control study.
Chair’s Message
Hello EUS-AAEM Section Members,
I am honored to be taking over the role of EUS-AAEM Section Chair. Under the leadership of Dr. Myers, the Section has continued to thrive over the past year. Successes include the Unmute Your Probe virtual lecture series, development of a Speaker’s Bureau to link medical school interest groups seeking point of care ultrasound education to interested residents, fellows and faculty, and a re-vamp of the Regional Ultrasound Course initiative to bring high quality, hands-on ultrasound education to practicing Physicians.
This year, the Section will continue to work to foster the professional development of our members and provide education regarding point of care ultrasound through expanded virtual content and in-person offerings. My goal is to cultivate opportunities for scholarship and engagement within the Section. Currently, we have an impressive membership of over 800 individuals. I hope to build connections between Section members, as well as develop collaborations with other Sections and groups, to enhance membership experience and further the objectives of the EUS.
Thank you for your membership and engagement in the Emergency Ultrasound Section. Please feel free to contact me at any point with any questions or to get involved by emailing me.
Allison Zanaboni, MD FAAEM
EUS-AAEM Chair, 2022-2023
Ultrasound Building Blocks
Point-of-Care Transorbital Ultrasound for the Diagnosis of Optic Neuritis: A Case Report
Nathaniel Leu, MD MS
Departments of Emergency Medicine and Emergency Ultrasound
Highland Hospital, Alameda Health System, Oakland, CA
Case:
A 27-year-old Female with a past medical history of hypothyroidism presented with a number of vague symptoms including fatigue, weakness, foggy thinking, slowed speech, intermittent headaches, and blurry vision. She states she feels weak intermittently and sometimes drops her toothbrush. The patient was seen for these same symptoms 1 month prior. She was found to have hypothyroidism and discharged on levothyroxine. She states that her symptoms are not improving and her vision has progressively worsened
Pertinent Physical Exam:
Eye Exam:
- Visual Acuity: Right eye 20/30, Left eye Counts fingers at 3 feet
- Pupils: Right eye round and reactive 4>2mm, Left eye round and reactive 4>3mm, + Relative afferent pupillary defect
- Extraocular movements: Full bilaterally, Left eye periorbital pain with extraocular movements
Neurologic Exam:
- Alert and oriented to person, place, time, situation
- Cranial nerves III-XII otherwise intact
- Normal sensation, motor, and reflexes
- Normal cerebellar function
Questions:
- What acute ocular conditions can be diagnosed and evaluated via transorbital ultrasound?
- What is the differential diagnosis of increased optic nerve sheath diameter?
Answers:
- Transorbital ultrasound is useful for diagnosis and evaluation of elevated intracranial pressure, optic neuritis, retinal detachment, choroidal detachment, vitreous detachment, vitreous hemorrhage, lens dislocation, retrobulbar hematoma, foreign bodies, globe ruptures, and pupillary defects.
- The differential diagnosis for increased optic nerve sheath diameter includes optic neuritis, glaucoma, and any diagnosis associated with elevated intracranial pressure, such as stroke, brain mass, intracranial hemorrhage, cerebral edema, hydrocephalus, or meningitis (5).
Case Discussion:
This patient underwent a transorbital bedside ultrasound using a linear array probe. Transverse and longitudinal orientations were scanned in detail and optic nerve sheath diameters (ONSD) were measured 3mm from the posterior globe margin. Right ONSD was 4.4mm and normal in appearance (Figure 1). Left ONSD was elevated at 7mm and grossly edematous (Figure 2).
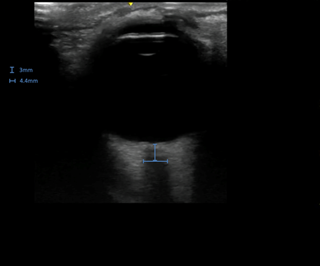
Figure 1. Transorbital ultrasound of the right orbit demonstrating normal optic nerve sheath diameter of 4.4mm
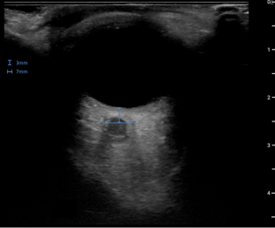
Figure 2. Transorbital ultrasound of the left orbit demonstrating an edematous and widened optic nerve sheath with a diameter of 7mm
The patient was admitted and received pulsed dose steroids. Brain MRI later demonstrated perineurial enhancement of the left optic nerve concerning for demyelinating optic neuritis (Figure 3). The patient later tested positive for anti-myelin oligodendrocyte glycoprotein optic neuritis.
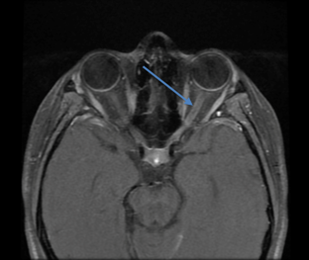
Figure 3. Magnetic resonance imaging of the brain demonstrating Fluid-attenuated inversion recovery (FLAIR) signal hyperintensity in the posterior left optic nerve (arrow) with associated hyperenhancement
Optic neuritis is an inflammatory demyelination of the optic nerve highly associated with multiple sclerosis. It typically presents in females aged 18-45 years (1) with symptoms including acute painful monocular vision loss over weeks to months often associated with a relative afferent pupillary defect. It is classically diagnosed through a dilated fundoscopic exam, although sensitivity may be as low as 35%. Magnetic resonance imaging (MRI) is the most sensitive test, but access to this imaging modality is often limited in the emergency department (2). Pulsed high-dose corticosteroid therapy is the treatment of choice, as it accelerates visual recovery and decreases long-term complications in select populations (1).
ONSD can be easily measured by bedside transorbital ultrasound by measuring the diameter at a distance of 3mm from the posterior globe margin. Lochner et al. (4) demonstrated that in patients with optic neuritis, median ONSD was thicker on the affected side (median 6.3 mm, interquartile range 6.0-6.5mm) compared to controls (median 5.2mm, interquartile range 4.8-5.5mm; p<0.001 (3). Some studies have suggested that the edematous appearance of the optic nerve is associated with more severe visual loss (4, 5, 6).
Transorbital ultrasound is useful for many diagnoses in the setting of acute monocular vision loss. It is a cost-effective, timely, noninvasive, and easily available test that may expedite workup for a previously undiagnosed patient with multiple sclerosis presenting with visual complaints. Due to the many limitations associated with brain/orbital MRI, bedside transorbital ultrasound may be useful to aid in the diagnosis of optic neuritis.
Clinical Pearls:
- Transorbital ultrasound is useful in the setting of acute monocular vision loss and may provide definitive diagnosis
- Optic nerve sheath diameter is measured 3mm from the posterior globe margin.
- Increased optic nerve sheath diameter over 5mm may suggest increased intracranial pressure or optic neuritis
References:
- The clinical profile of optic neuritis: experience of the Optic Neuritis Treatment Trial. Optic Neuritis Study Group. Arch Ophthalmol 1991;109:1673-8
- Balcer LJ. Optic neuritis. N Engl J Med 2006;354:1273-80
- Lochner P, Cantello R, Fassbender K, et al. Longitudinal assessment of transorbital sonography, visual acuity, and biomarkers for inflammation and axonal injury in optic neuritis. Dis Markers 2017;2017: 5434310.
- Dees C, Buimer R, Dick AD, Atta HR. Ultrasonographic investigation of optic neuritis. Eye 1995;9:488-94.
- Saigh, Mark Philip, et al. “Acute Optic Neuritis Diagnosed by Bedside Ultrasound in an Emergency Department.” The Journal of Emergency Medicine, vol 57, no.2,2019,pp.207-211.
- Lochner P, Leone MA, Coppo L, et al. B-mode transorbital ultrasonography for the diagnosis of acute optic neuritis. A systemic review. Clin Neurophysiol 2016;127:803-9
Resident Section
Hidden in Plain Sight: Impending Paradoxical Embolism
Megan Winters, DO, Patrick Charles, DO, Michael Ullo, MD
Introduction:
Shortness of breath is a common chief complaint in the emergency department and has a vast differential that can be attributed to pathology in single or multiple organ systems. Pulmonary embolism (PE) is a frequently encountered diagnosis in the emergency department, and is the third most common cause of death from cardiovascular disease after heart attack and stroke [1]. Point-of-care-ultrasound (POCUS) has become an important diagnostic tool in the evaluation of undifferentiated dyspnea. Common ultrasound findings seen in PE include right heart dilation, septal bowing, and right ventricular free wall hypokinesis [3]. Though it is rare, direct visualization of a right heart thrombus in transit is a unique finding that is highly suggestive of PE [2].
Case Report:
An elderly female with a history of stroke, hypertension, and diabetes presented to the emergency department after being found on the ground by family. Prior to falling the patient felt lightheaded and short of breath. She reported worsening dyspnea and left flank pain for two weeks.
On arrival the patient was afebrile with a heart rate of 77 beats per minute and oxygen saturation of 92% on room air. She was normotensive and mildly tachypneic. Exam revealed a cachectic female with clear lungs and pitting edema of her lower extremities.
Due to the patient’s hypoxia and dyspnea, a cardiac POCUS study was obtained which demonstrated a large right atrial thrombus with right ventricular dilation on the apical four chamber view (Figure 1). Parasternal short axis view revealed flattening of the interventricular septum, also known as the “D” sign, indicating elevated right ventricular pressures.
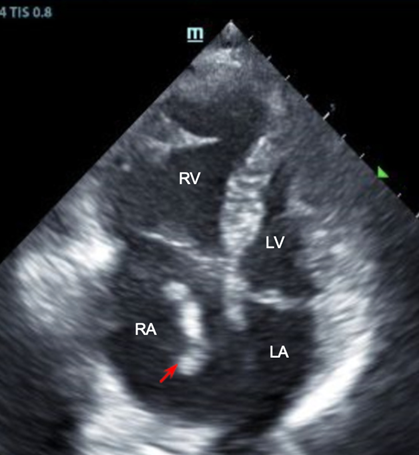
Figure 1. Right heart thrombus in transit. Apical four chamber view, cardiac ultrasound. Thrombus (red arrow) in transit identified in the right atrium with associated right ventricular dilation.
Given the heightened suspicion for thromboembolism, a CT angiogram of the chest, abdomen, and pelvis was obtained. Angiography revealed extensive clot burden, including a right pulmonary artery thrombus, thrombus of the celiac artery, and a left renal artery thrombus causing left renal infarct (Figures 2 and 3).Subsequent transthoracic echocardiography performed by the radiology technician demonstrated biatrial thrombi straddling a presumed patent foramen ovale (PFO) (Figures 4 and 5).
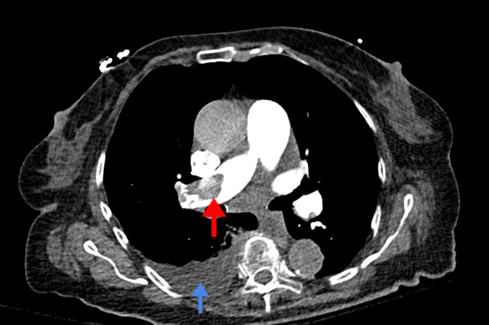
Figure 2. Pulmonary embolism. CT angiogram. Large right main pulmonary artery embolism (red arrow) with trace right pleural effusion (blue arrow).
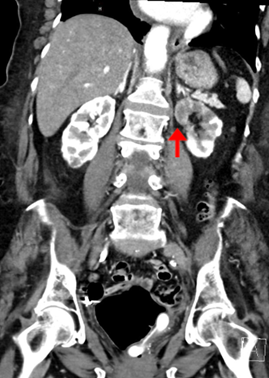
Figure 3. Left renal infarct. CT angiogram. A left renal infarct is present (red arrow).
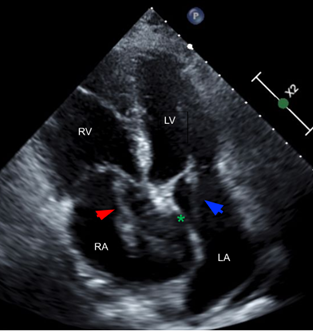
Figure 4. Impending paradoxical embolism. Apical four chamber view, cardiac ultrasound. Right atrial thrombus (red arrow), patent foramen ovale (green asterisk), and left atrial thrombus (blue arrow).
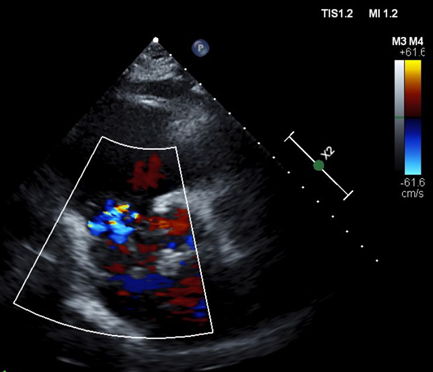
Figure 5. Patent foramen ovale. Echocardiogram. Mixing of right and left atrial blood through patent foramen ovale. Left atrial thrombus denoted by yellow arrow.
Due to the extensive clot burden, both cardiothoracic and vascular surgery were emergently consulted. The patient subsequently underwent successful pulmonary embolectomy with thrombectomy and PFO repair. She was discharged to subacute rehabilitation after a three-week hospitalization.
Discussion:
Direct visualization of a right atrial thrombus on POCUS is highly suggestive of PE, and can carry a mortality rate of approximately 30% [4]. PFO has an estimated prevalence of 25% in the general population and is an incidental finding without clinical significance in most cases [11]. In rare circumstances, a PFO can allow a right heart thrombus in transit to move into the left heart and potentially lead to paradoxical embolization of the systemic vasculature, as was likely the case with this patient’s renal infarct and celiac artery thrombus [2]. This phenomenon is referred to as an impending paradoxical embolism (IPDE) [5].
IPDE carries a high 24-hour and in-hospital mortality rate [6, 7]. If IPDE is identified, further diagnostics should be utilized to determine the extent of the clot burden. Increased right-sided pressures can result in right-to-left transit of thrombi and subsequent fragmented embolization in the cerebral and visceral circulation [8].
IPDE can be treated with systemic anticoagulation, fibrinolytics, catheter-directed thrombolysis, or surgical thrombectomy. However, no clear consensus currently exists for management of this condition [9]. Prior research has demonstrated greater mortality and morbidity with thrombolysis due to fragmented embolization and suggests that stable patients should be treated surgically [9, 10]. Patients with hemodynamic instability who cannot tolerate surgery should be considered for thrombolysis [9, 10].
References
- Goldhaber SZ, et al. Pulmonary embolism and deep vein thrombosis. Lancet. 2012 May 12’379(9828):1835-46. Doi: 10.1016/S0140-6736(11)61904-1. Epub 2012. Apr 10. PMID: 22494827
- Bonanni L, et al. Fluttering thrombus in patent foramen ovale with paradoxical and cerebral embolism. Circulation 2014;129:e343–4.
- Ma, Christopher M., et al. Right Ventricular Dysfunction and the “D”-shaped Left Ventricle. (2020) Anesthesiology. 132 (1): 155.
- Kalivoda EJ, et al. Right Heart Thrombus in Transit Diagnosed With POCUSed Cardiac Ultrasound in the Emergency Department. Cureus. 2020;12(7):e9354. Published 2020 Jul 23.
- Zoltowska DM, et al. Serpentine thrombus in the heart: a rare case of trapped thrombus in patent foramen ovale. Case Reports 2018;2018:bcr-2017-223469
- Rose PS, et al. Treatment of right heart thromboemboli. Chest 2002;121:806–14
- Chartier L, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999;99:2779–83.
- Konstantinides S, et al. Patent foramen ovale is an important predictor of adverse outcome in patients with major pulmonary embolism. Circulation 1998;97:1946–51.
- Seo WW, et al. Systematic Review of Treatment for Trapped Thrombus in Patent Foramen Ovale. Korean Circ J. 2017 Sep;47(5):776-785.
- Fauveaua E, et al. Surgical or medical treatment for thrombus straddling the patent foramen ovale: Impending paradoxical embolism? Report of four clinical cases and literature review. Archives of Cardiovascular Diseases. 2008;101(10):637–644.
- Hagen PT, et al. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 1984;59:17–20.
Fellow Section
Use of Resuscitative Transesophageal Echocardiography in the Emergency Department to Diagnose Pulmonary Embolism
Caresse Vuong, MD, Aimee Vos, MD and Katharine M Burns, MD
Introduction:
Resuscitation of patients who present in cardiac arrest is one of the most challenging clinical scenarios faced by emergency medicine physicians. Updated in 2020, the American Heart Association Guidelines for Cardiopulmonary Resuscitation continues to recommend the use of transthoracic echocardiography (TTE) as a potential adjunct to identify reversible causes of cardiac arrest.1 Rapid recognition of echo findings such as tamponade or right heart strain associated with massive pulmonary embolism can significantly impact outcomes of resuscitation.2-4 TTE has also been shown to predict survivability by differentiating organized cardiac activity from cardiac standstill.4-6 However, TTE has several limiting factors during an arrest setting, including poor quality views due to body habitus, obstruction of views from ongoing chest compressions or defibrillation pads, and the potential to prolong pulse check durations.7-8
Transesophageal echocardiography (TEE) is emerging as a tool that can overcome these challenges, providing reliable diagnostic insight in arrest settings without compromising quality chest compressions and resuscitation. The TEE probe is placed in the esophagus post-intubation, such that its proximity to the heart provides high-quality images unencumbered by subcutaneous fat or gastric insufflation. This enhances diagnostic capability for reversible causes of arrest, allows for assessment of intravascular status, and guides procedures as well.9 With a continuous view of cardiac activity during chest compressions, rather than in between chest compressions, TEE also improves quality of resuscitation by offering real-time feedback. As this case will illustrate, TEE can guide resuscitative management in a critically ill, arresting patient, and rapidly identify the underlying diagnosis.
Case Report:
A 72-year-old female with history of COVID pneumonia two months prior presented to the emergency department with shortness of breath. Per EMS report, the patient was tachycardic with a heart rate of 120’s, hypoxic to 70% on room air, and increasingly lethargic en route. In the emergency department, the patient arrived in a peri-arrest state, with altered mental status, oxygen saturation 93% on non-rebreather, and heart rate in the 30s. Patient lost pulses and compressions were started with narrow complex pulseless electrical activity (PEA) on the monitor. She had return of spontaneous circulation (ROSC) after several minutes of CPR and was subsequently intubated. Throughout the resuscitation the patient repeatedly lost pulses, with ROSC regained within 1-2 rounds of compressions each time. A bedside TTE was performed which suggested right ventricular enlargement, however there was very poor visualization due to body habitus (Image 1).
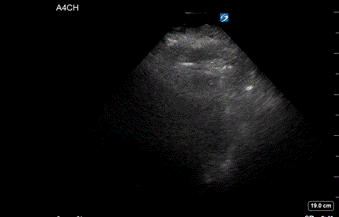
Image 1: Bedside transthoracic echocardiography (TTE): Apical 4 Chamber view. Poor visualization of cardiac structures.
A bedside TEE was performed to direct further resuscitation. TEEs performed in the critical care and emergency department settings often involve four main resuscitative views: midesophageal four chamber, midesophageal long axis, transgastric short axis, and midesophageal bicaval view.10 Two of these four views are described below:
- The Mid-esophageal Four Chamber view (similar to TTE Apical 4 Chamber) revealed a severely enlarged right atrium and ventricle with septal bowing and strain (Image 2).
- The Transgastric Short Axis view (similar to TTE Parasternal Short) also depicted a severely enlarged right ventricle with septal flattening (Image 3)
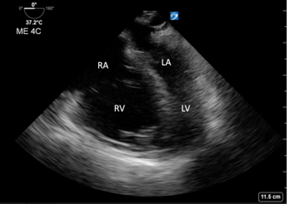
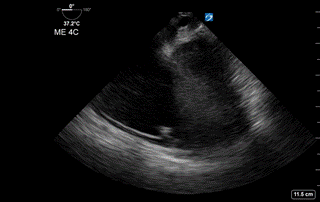
Image 2: Transesophageal echocardiography (TEE): Mid-esophageal 4 Chamber view. Right ventricle and atrium are dilated with septal bowing.
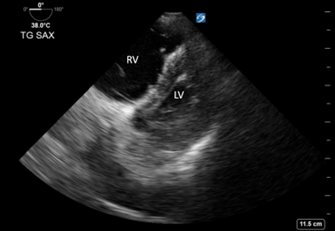
Image 3: Transesophageal echocardiography (TEE): Transgastric Short Axis view. Right ventricle is dilated with septal flattening. “D sign” indicates right heart strain.20
With evidence of right heart strain, an additional TEE view was obtained in order to look for a pulmonary embolism:
- The Mid-esophageal Ascending Aorta Short Axis view visualized a saddle embolism extending into the right and left pulmonary arteries. (Image 4, Image 5)
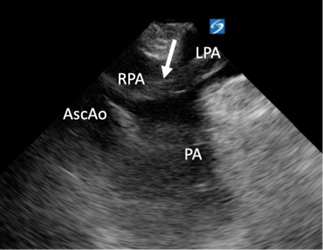
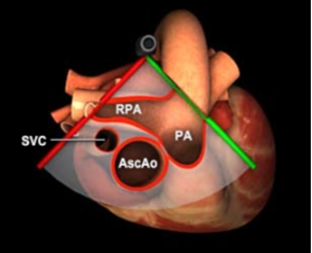
Image 4: Transesophageal echocardiography (TEE): Mid-esophageal Ascending Aorta Short Axis view. Arrow points to a clot that extends between the right and left pulmonary arteries. 3D graphic obtained from the Toronto General Hospital Department of Anesthesia Virtual Transesophageal Echocardiography website.21
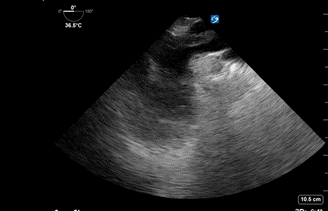
Image 5: Transesophageal echocardiography (TEE): Mid-esophageal Ascending Aorta Short Axis View with visualization of clot.
The patient was treated for massive pulmonary embolism whereby a 50 mg bolus of tPA was pushed over 1 minute with subsequent ROSC; the remaining 50 mg tPA was given over 1 hour. Cardiovascular surgery was consulted and within an hour they took the patient to the operating room for an emergent pulmonary embolectomy. The patient was placed on cardiopulmonary bypass and the surgeon evacuated large clot burdens from the main pulmonary artery and right pulmonary artery.
Discussion:
Saddle pulmonary embolism has a high mortality rate, but there are improved outcomes if diagnosis and treatment are initiated early. In an observational study evaluating the effectiveness of pulmonary embolism response teams, it was independently found that the risk of mortality was reduced by 5% for each hour earlier the diagnosis was made.11 In this particular case, TEE took approximately five minutes to reveal a saddle pulmonary embolism once the probe was placed. Within ten minutes the patient received her first bolus of tPA and within one hour the patient was in the OR undergoing a life-saving procedure. TEE was critical in hastening this patient’s care.
This case exemplifies how TEE is a vital bedside diagnostic tool for critically ill patients. Although CT pulmonary angiogram is the gold standard for diagnosis of pulmonary embolism, this would be difficult to obtain for a patient being actively resuscitated. As this patient suffered repeated cardiac arrests, she was never stable enough to leave the resuscitation bay, let alone survive a scan which can take ~30 minutes including transport and set-up. In a retrospective study of 1246 cardiac arrest cases over 8 years, 60 (4.8%) were caused by pulmonary embolism, with 24 (40%) cases diagnosed by TTE (n=6) or TEE (n=18). Only 5 cases (8.3%) were diagnosed with CT pulmonary angiogram, with the radiologic study taking place before cardiac arrest or after stabilization.12
Several studies have shown how TEE can be influential in guiding decisions in a resuscitation.13-15 In a four-year retrospective observational study, 38 critical care intensivists completed 274 consecutive TEE studies, of which common indications included: hemodynamic instability (45.2%) and cardiac arrest (20.1%). In 79.5% of these cases, TEE findings prompted a proposal for change in patient management.13 Although proficiency in TEE may seem daunting, a study by Artfield et al has shown that after a 4-hour workshop, emergency physicians can retain competence in a 4-view focused protocol.10 As such, it is manageable to use TEE during resuscitations in an emergency department with sufficient training. In a prospective observational study of 33 out-of-hospital cardiac arrests, emergency physicians performed intra-arrest TEE using a 4-view protocol. In 32/33 cases (97%), TEE provided findings of diagnostic, therapeutic, or prognostic value (i.e. fine ventricular fibrillation prompting defibrillation, intracardiac thrombus prompting thrombolysis, etc.)15
As demonstrated in this case study, TEE has potential to improve resuscitation by offering real-time feedback for chest compressions. In a study by Hwang et al, hand placement on the chest that produced maximal compression near the left ventricular outflow tract (LVOT) or aorta resulted in a narrowing of the LVOT and decreased stroke volume,16 which can have detrimental effects on maintenance of circulation. Additionally, a study by Catena et al found that an open LVOT during CPR was associated with successful resuscitations.17 In a randomized control trial with swine models, TEE was utilized to optimize maximal compression over the left ventricle, which improved hemodynamics and enhanced survival.18 Finally, TEE has also been shown to shorten pauses in chest compressions when compared to TTE19, further optimizing resuscitation.
Conclusion:
Transesophageal echocardiography has exceptional potential as a bedside tool for peri-arrest, critically ill patients by providing reliable, continuous views that guide resuscitative decisions and increase effectiveness of chest compressions.
References
- Merchant RM, Topjian AA, Panchal AR, Cheng A, Aziz K, Berg KM, Lavonas EJ, Magid DJ; on behalf of the Adult Basic and Advanced Life Support, Pediatric Basic and Advanced Life Support, Neonatal Life Support, Resuscitation Education Science, and Systems of Care Writing Groups. Part 1: executive summary: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(suppl 2):S337–S357.
- Weekes AJ, Thacker G, Troha D, Johnson AK, Chanler-Berat J, Norton HJ, Runyon M. Diagnostic Accuracy of Right Ventricular Dysfunction Markers in Normotensive Emergency Department Patients With Acute Pulmonary Embolism. Ann Emerg Med. 2016 Sep;68(3):277-91.
- Alpert EA, Amit U, Guranda L, Mahagna R, Grossman SA, Bentancur A. Emergency department point-of-care ultrasonography improves time to pericardiocentesis for clinically significant effusions. Clin Exp Emerg Med. 2017 Sep 30;4(3):128-132
- Gaspari R, Weekes A, Adhikari S, Noble VE, Nomura JT, Theodoro D, Woo M, Atkinson P, Blehar D, Brown SM, Caffery T, Douglass E, Fraser J, Haines C, Lam S, Lanspa M, Lewis M, Liebmann O, Limkakeng A, Lopez F, Platz E, Mendoza M, Minnigan H, Moore C, Novik J, Rang L, Scruggs W, Raio C. Emergency department point-of-care ultrasound in out-of-hospital and in-ED cardiac arrest. Resuscitation. 2016 Dec;109:33-39.
- Salen P, Melniker L, Chooljian C, Rose JS, Alteveer J, Reed J, Heller M. Does the presence or absence of sonographically identified cardiac activity predict resuscitation outcomes of cardiac arrest patients? Am J Emerg Med. 2005 Jul;23(4):459-62.
- Blaivas M, Fox JC. Outcome in Cardiac Arrest Patients Found to Have Cardiac Standstill on the Bedside Emergency Department Echocardiogram. Acad Emerg Med 2001;8:616-21.
- Clattenburg EJ, Wroe P, Brown S, Gardner K, Losonczy L, Singh A, Nagdev A. Point-of-care ultrasound use in patients with cardiac arrest is associated prolonged cardiopulmonary resuscitation pauses: A prospective cohort study. Resuscitation. 2018 Jan;122:65-68.
- Huis In 't Veld MA, Allison MG, Bostick DS, Fisher KR, Goloubeva OG, Witting MD, Winters ME. Ultrasound use during cardiopulmonary resuscitation is associated with delays in chest compressions. Resuscitation. 2017 Oct;119:95-98.
- Fair J, Mallin M, Mallemat H, Zimmerman J, Arntfield R, Kessler R, Bailitz J, Blaivas M. Transesophageal Echocardiography: Guidelines for Point-of-Care Applications in Cardiac Arrest Resuscitation. Ann Emerg Med. 2018 Feb;71(2):201-207.
- Arntfield, Robert, et al. “Focused Transesophageal Echocardiography for Emergency Physicians-Description and Results from Simulation Training of a Structured Four-View Examination.” Critical Ultrasound Journal, vol. 7, no. 1, Dec. 2015, p. 27
- Wright C, Goldenberg I, Schleede S, McNitt S, Gosev I, Elbadawi A, Pietropaoli A, Barrus B, Chen YL, Mazzillo J, Acquisto NM, Van Galen J, Hamer A, Marinescu M, Delehanty J, Cameron SJ. Effect of a Multidisciplinary Pulmonary Embolism Response Team on Patient Mortality. Am J Cardiol. 2021 Dec 15;161:102-107.
- Kürkciyan I, Meron G, Sterz F, et al. Pulmonary Embolism as Cause of Cardiac Arrest: Presentation and Outcome. Arch Intern Med. 2000;160(10):1529–1535.
- Arntfield R, Lau V, Landry Y, Priestap F, Ball I. Impact of Critical Care Transesophageal Echocardiography in Medical-Surgical ICU Patients: Characteristics and Results From 274 Consecutive Examinations. J Intensive Care Med. 2020 Sep;35(9):896-902.
- Arntfield R, Pace J, Hewak M, Thompson D. Focused Transesophageal Echocardiography by Emergency Physicians is Feasible and Clinically Influential: Observational Results from a Novel Ultrasound Program. J Emerg Med. 2016 Feb;50(2):286-94.
- Teran F, Dean AJ, Centeno C, Panebianco NL, Zeidan AJ, Chan W, Abella BS. Evaluation of out-of-hospital cardiac arrest using transesophageal echocardiography in the emergency department. Resuscitation. 2019 Apr;137:140-147.
- Hwang SO, Zhao PG, Choi HJ, Park KH, Cha KC, Park SM, Kim SC, Kim H, Lee KH. Compression of the left ventricular outflow tract during cardiopulmonary resuscitation. Acad Emerg Med. 2009 Oct;16(10):928-33.
- Catena E, Ottolina D, Fossali T, Rech R, Borghi B, Perotti A, Ballone E, Bergomi P, Corona A, Castelli A, Colombo R. Association between left ventricular outflow tract opening and successful resuscitation after cardiac arrest. Resuscitation. 2019 May;138:8-14.
- Anderson KL, Castaneda MG, Boudreau SM, Sharon DJ, Bebarta VS. Left Ventricular Compressions Improve Hemodynamics in a Swine Model of Out-of-Hospital Cardiac Arrest. Prehosp Emerg Care. 2017 Mar-Apr;21(2):272-280.
- Fair J 3rd, Mallin MP, Adler A, Ockerse P, Steenblik J, Tonna J, Youngquist ST. Transesophageal Echocardiography During Cardiopulmonary Resuscitation Is Associated With Shorter Compression Pauses Compared With Transthoracic Echocardiography. Ann Emerg Med. 2019 Jun;73(6):610-616.
- Rudski, Lawrence G et al. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: a Report from the American Society of Echocardiography Endorsed by the European Association of Echocardiography, a Registered Branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. Journal of the American Society of Echocardiography. 23.7 (2010): 685–713
- Corrin M, Meineri M, Vegas A, Tait G. (2020). TEE Standard Views module. Virtual Transesophageal Echocardiography. Toronto General Hospital Department of Anesthesia. http://pie.med.utoronto.ca/tee/TEE_content/TEE_standardViews_intro.html
Ultrasound Guided Procedures
Ultrasound Guided Ganglion Cyst Aspiration from the Dorsum of the Wrist
Alexis Avellino, MD and Michael Rosselli, MD MPH
Ganglion cysts are among the most common causes of soft tissue swelling to the hand and wrist. Although benign, they may cause symptoms ranging from localized pain to loss of function. The most common place for ganglion cysts to occur is in the dorsum of the wrist (~60-70%) and have a higher incidence in females (43/100,000) than in males (25/100,000). Those located in this region usually originate from the radiolunate ligament via a pedicle or stalk.
Aspiration of these cysts is a relatively quick procedure that provides almost immediate symptomatic relief. Traditionally, blind aspirations of ganglion cysts have been a less invasive way of obtaining symptomatic pain relief as compared to surgery. However with the blind approach, successful aspiration is lower and more painful as compared to the utilization of point of care ultrasound (POCUS). POCUS utilizes in-plane needle guidance to allow for a more successful and ultimately less painful aspiration. A study published in Acta Radiologica: SAGE Journal revealed that US-guided aspiration is a safe and potentially effective treatment for ganglion cysts of the wrist, with high patient satisfaction.
Scanning Technique:
Using a small footprint linear transducer placed to the dorsum of the wrist joint in either the short axis or long axis orientation (see Figure 1/1a), search for the cystic structure. It usually appears as a well-circumscribed, anechoic or hypoechoic structure that may demonstrate internal septations (Figure 2). Utilize color flow doppler on ultrasound to ensure the structure is indeed a cystic structure (negative color flow) and is not a blood vessel (positive color flow). To optimize ultrasound visualization, place an object under the wrist so that the wrist is held in slight flexion.
Procedure:
Once the appropriate structure is identified, mark the area in which you intend to insert your needle. Thoroughly clean the designated area with chlorhexidine. After doing so, anesthetize the skin using local anesthesia or ethyl chloride spray and reorient your small footprint, linear transducer probe. Insert an 18 gauge needle attached to a 3-5cc syringe using an in-plane approach as demonstrated in Figure 1/1a. Locate the needle tip and advance until you reach the well-defined cystic structure (Figure 2). After you have punctured the capsule, aspirate the cystic contents which should appear as a gelatinous material. Immediate symptomatic relief is often obtained after aspiration.
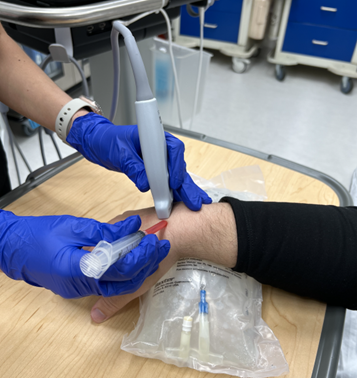
Figure 1: Transducer and needle position for transverse axis in plane US guided dorsal wrist ganglion cyst aspiration
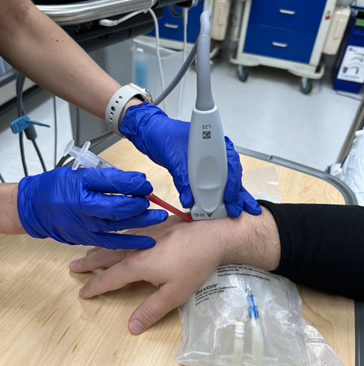
Figure 1a: Transducer and needle position for long axis in plane US guided dorsal wrist ganglion cyst aspiration
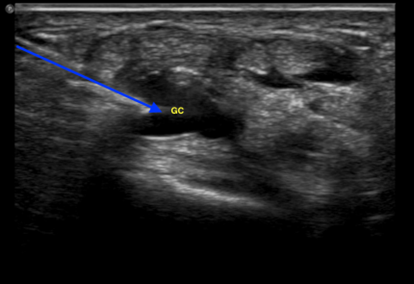
Figure 2: Ultrasound view of an in plane ganglion cyst needle aspiration from the dorsum of the wrist. Blue arrow: Needle path; Yellow “GC”: Ganglion cyst
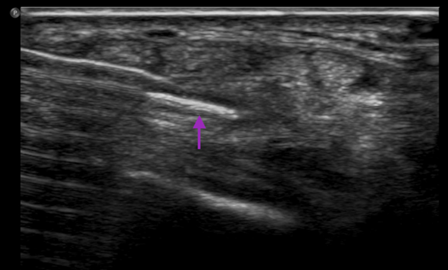
Figure 3: Ultrasound image of an in plane needle view after ganglion cyst aspiration from the dorsum of the wrist. Purple arrow: Needle
References
- De Keyser, Filip. “Ganglion Cysts of the Wrist and Hand.” UpToDate, https://www.uptodate.com/contents/ganglion-cysts-of-the-wrist-and-hand?search=ganglion%20cyst%20wrist&usage_type=default&source=search_result&selectedTitle=1~150&display_rank=1.
- Gude, Warren, and Vincent Morelli. “Ganglion Cysts of the Wrist: Pathophysiology, Clinical Picture, and Management.” Current Reviews in Musculoskeletal Medicine, Humana Press Inc, Dec. 2008, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2682407/.
- Zeidenberg, J; Aronowitz, JG; Landy, DC; Owens, PW; Jose, J; “Ultrasound-Guided Aspiration of Wrist Ganglions: A Follow-up Survey of Patient Satisfaction and Outcomes.” Acta Radiologica (Stockholm, Sweden : 1987), U.S. National Library of Medicine, 7 Aug. 2015, https://pubmed.ncbi.nlm.nih.gov/26253928/.
Ultrasound Saves
TampoNOT: Use of POCUS to differentiate a mistaken case of acute cardiac tamponade
Liane McAuliffe, MD LT MC USN and Adrianna Kyle, MD LCDR MC USN
Case Presentation:
63-year-old male with history of hypertrophic cardiomyopathy (HCM), and end-stage interstitial lung disease (ILD) on home O2 of 3L, presented to emergency department from the Cardiology Clinic with concern for cardiac tamponade, after large pericardial effusion was identified on routine echocardiogram. He arrived at the clinic with SPO2 in mid 80s on his home O2 (baseline 92-94%). The patient reported three months of progressive dyspnea on exertion, and increased fatigue, worse than the past few days. He was currently undergoing cardiac evaluation for lung transplant for ILD and was taking aspirin, disopyramide, and verapamil for management of his HCM.
Vital sign included: T 36.5°C; HR-109; BP-156/100; RR-36; SPO2-87%; on 5L simple face mask. Patient was alert and oriented x3, cachectic and anxious appearing, with conversational dyspnea. Pulmonary exam was notable for inspiratory crackles in the bilateral lung bases and clubbing of fingernails. He was tachycardic with a 3/6 holosystolic murmur best heard at right lower sternal border. Two centimeters of jugular venous distention (JVD) was noted. CBC, CMP, troponin, NT-proBNP were unchanged from patient’s baseline.
POCUS demonstrated a large, circumferential pericardial effusion, and severe HCM with left ventricular hypertrophy (LVH). The inferior vena cava (IVC) was less than 2cm with close to 50% respiratory variability. POCUS also showed severe right ventricular hypertrophy (RVH) and a dilated RA with no collapse of RA or RV.
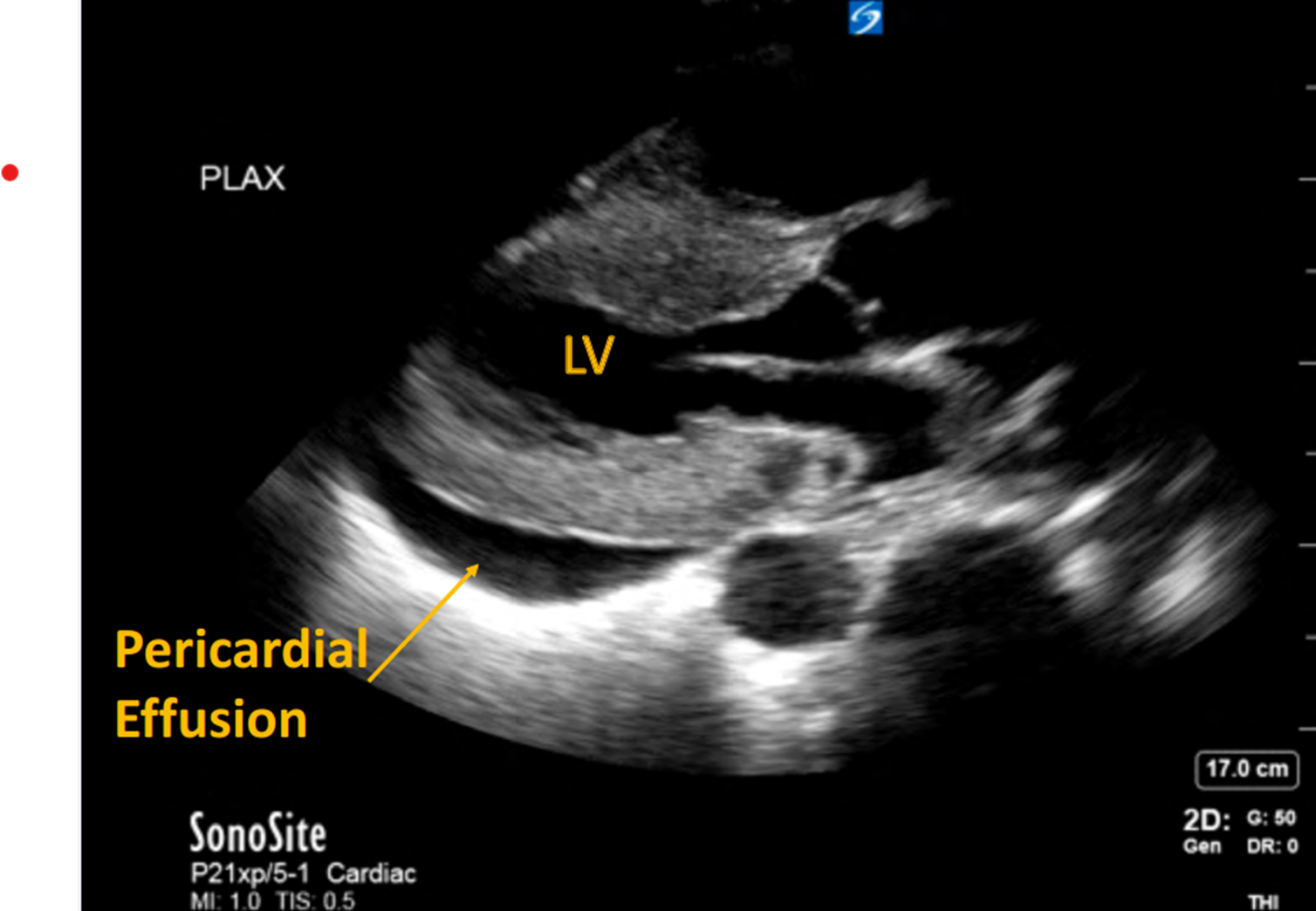
Image 1: Circumferential pericardial effusion. Severe hypertrophic cardiomyopathy of LV.
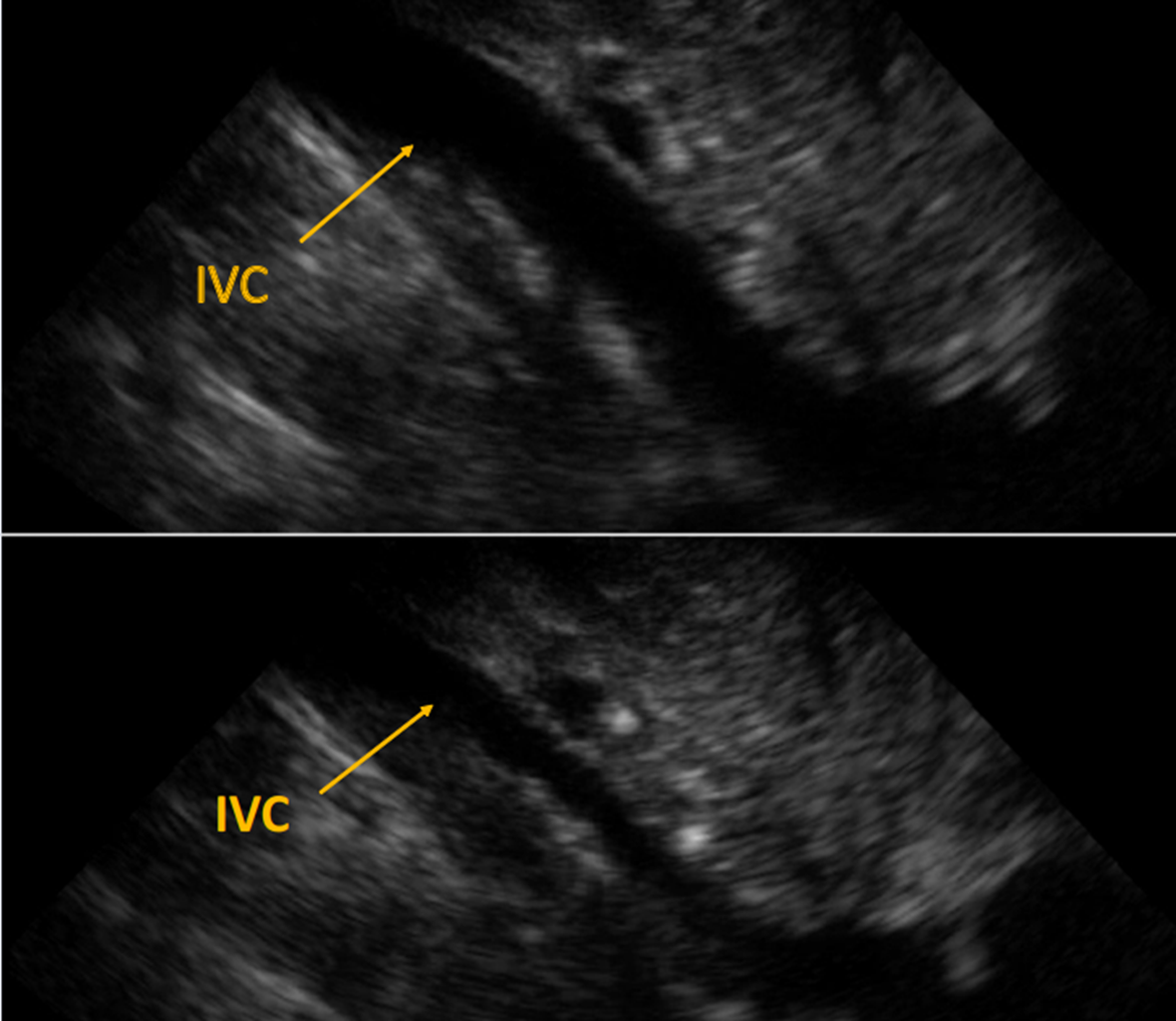
Image 2: Upper image IVC on expiration. Lower image IVC on inspiration Demonstrates close to normal respiratory variability.
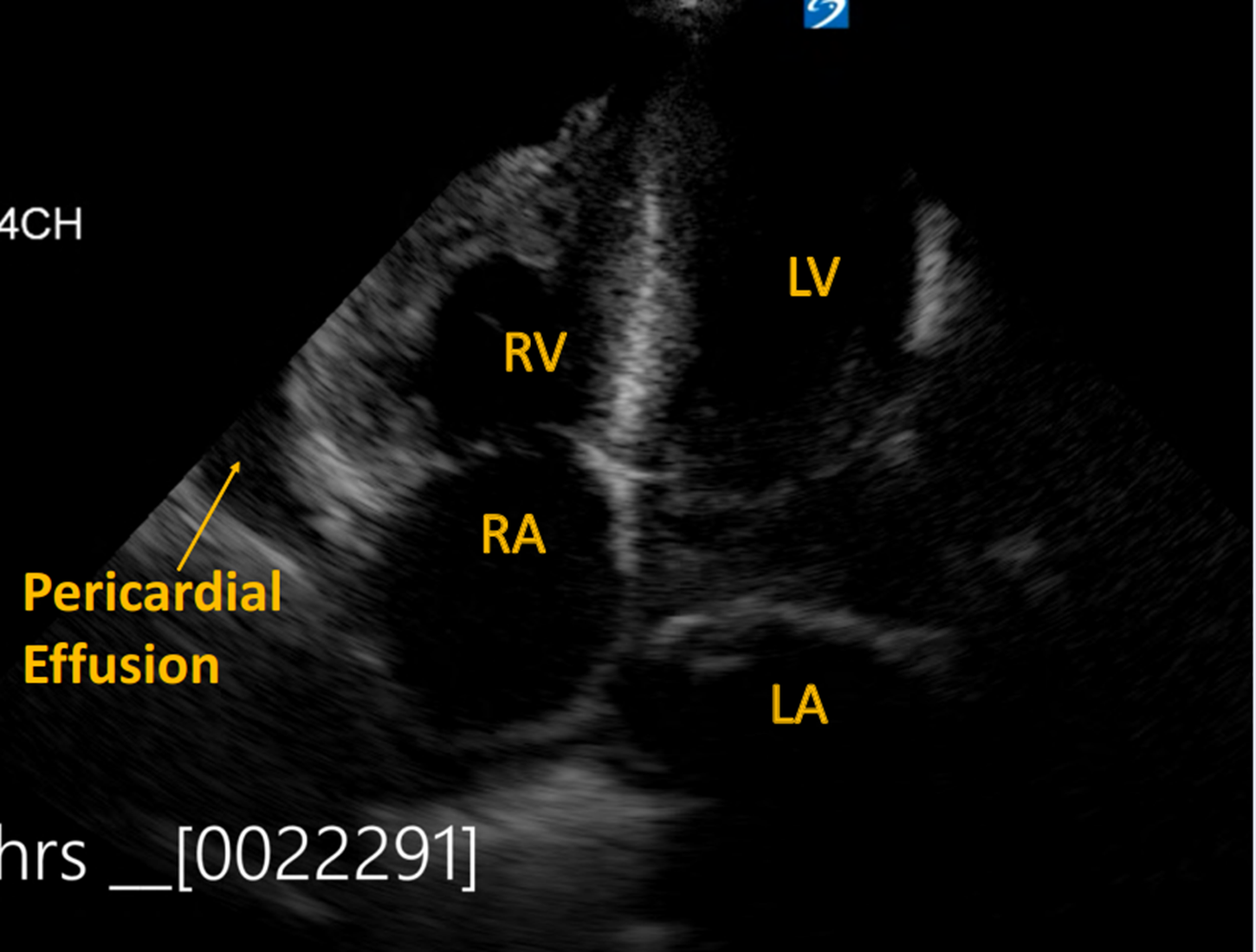
Image 3: Pericardial effusion present with RVH and dilated RA. No collapse of RA or RV.
Case Discussion:
Point of care ultrasound (POCUS) showed a large pericardial effusion without RA or RV collapse. While the IVC did not compress less than 50% with inspiration, the slight decrease was felt to be due to worsening of his chronic lung disease. The findings overall were not consistent with tamponade, but with a compensated physiology and thus no emergent intervention were performed. He remained normotensive and dyspnea improved with increased oxygen, eventually titrated to 5L. Interventional Cardiology was consulted, and they concurred that the effusion was chronic, and no emergent intervention was recommended. The patient was monitored overnight and discharged home the next day.
Pericardial tamponade is a medical emergency resulting from fluid accumulation in the pericardial sac which ultimately leads to decreased venous return and, subsequently, decreased cardiac output. If untreated, it is rapidly, and universally fatal. Point of care ultrasound (POCUS) is a rapid and accurate diagnostic tool with high sensitivity and specificity for identifying pericardial effusions and tamponade physiology.
Tamponade is a clinical diagnosis based on the patient’s presentation, hemodynamics, and echocardiographic findings. The classic findings of Beck’s triad (hypotension, muffled heart sounds, and JVD) are only seen in 40-50% of patients, and thus, is not an accurate predictor of tamponade.1,2 POCUS findings include presence of pericardial effusion, diastolic RV collapse, systolic RA collapse, and a plethoric IVC with minimal respiratory variability.2 Increased afterload on the RV in the setting of long-standing PAH resulted in RV hypertrophy and decreased compliance. In the setting of a moderate to large pericardial effusion, the decreased compliance and increased pressure in the right heart may decrease the tendency of these chambers to collapse during diastole.3 As a result, this may provide protective benefit against tamponade physiology. Because of this protective effect, it has been suggested that use of pulmonary vasodilators should be used with extreme caution in patients with PAH and compensated pericardial effusion.3
Take Home Points:
- Cardiac tamponade is a clinical diagnosis based on patient presentation, hemodynamics, and findings on echocardiogram and without evidence of instability, interventions should be undertaken with caution.
- The rate of fluid accumulation rather than the volume of fluid is more prognostic of impending tamponade.
- If the IVC does not appear plethoric and there is respiratory variation, tamponade is highly unlikely.
- Severe PAH with RVH may confer protective effect against tamponade physiology from pericardial effusions and clinicians should exercise extreme caution before aggressively treating the PAH or performing pericardiocentesis in these patients.
Disclaimer:
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government. We are military service members. This work was prepared as part of my official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties.
References:
- Hanson MG, Chan B. The role of point-of-care ultrasound in the diagnosis of pericardial effusion: a single academic center retrospective study. Ultrasound J. 2021 Feb 4;13(1):2. doi: 10.1186/s13089-021-00205-x. PMID: 33538920; PMCID: PMC7862446.
- Jacob S, Sebastian JC, Cherian PK, Abraham A, John SK. Pericardial effusion impending tamponade: a look beyond Beck's triad. Am J Emerg Med. 2009 Feb;27(2):216-9. doi: 10.1016/j.ajem.2008.01.056. PMID: 19371531.
- Alerhand S, Carter JM. What echocardiographic findings suggest a pericardial effusion is causing tamponade? Am J Emerg Med. 2019 Feb;37(2):321-326. doi: 10.1016/j.ajem.2018.11.004. Epub 2018 Nov 17. PMID: 30471929.
- Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade. J Emerg Trauma Shock. 2012 Jan;5(1):72-5. doi: 10.4103/0974-2700.93118. PMID: 22416160; PMCID: PMC3299160.
- Khan MU, Khouzam RN. Protective effect of pulmonary hypertension against right-sided tamponade in pericardial effusion. South Med J. 2015 Jan;108(1):46-8. doi: 10.14423/SMJ.0000000000000230. PMID: 25580757.
POCUS for Ocular Trauma
Sidrah Syed, MD and Timothy Stokes, MD
Case Presentation:
A 60-year-old female with a past medical history of alcohol abuse presented to the emergency department after an assault. She reported that her boyfriend pushed her down, which caused her to hit her face on the corner of the front porch. She complained of a headache and face pain on the left side associated with blurry vision in the left eye. Further history was limited as the patient was clinically intoxicated and admitted to alcohol use prior to the visit.
On physical exam, the patient appeared intoxicated with slurred speech. She had scattered abrasions over the left side of her face but minimal tenderness to palpation of the facial bones and minimal swelling. Her ocular exam revealed pupils that were equal and reactive to light, extraocular eye movements were intact, and conjunctiva were normal. Her visual acuity however was 20/30 in the right eye and the left eye was only able to visualize finger waving. Staining of the eye revealed a negative Seidel Test. Intraocular pressures were normal.
After the marked decrease in visual acuity was discovered, the patient was able to clarify that her blurry vision also included a component of double vision. Covering her right eye did not make the diplopia resolve, however covering the left eye did cause resolution of her diplopia. Given the monocular diplopia, an ocular pathology was suspected over an intracranial one and ultrasound was used to further evaluate the globe (Figure 1).
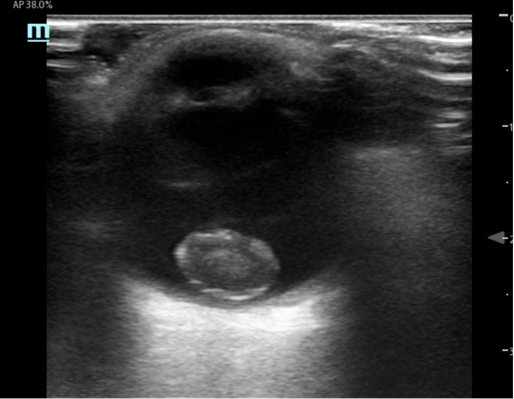
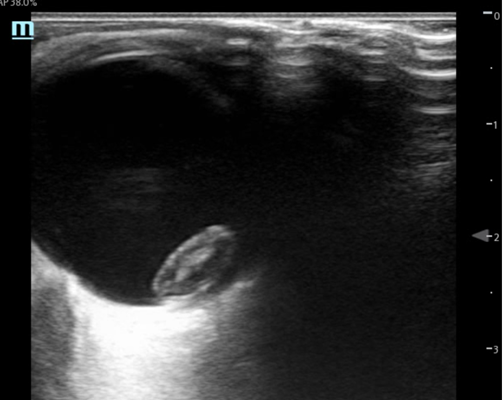
Figure 1: Transverse and Sagittal stills from the ocular ultrasound of patient’s left eye
Ocular ultrasound demonstrated a mixed echogenicity ovoid structure in the posterior globe consistent with a lens dislocation. Contrary to the normal lens position, the lens was seen en face. There were no signs of vitreous hemorrhage, globe rupture, retinal detachment, or other traumatic abnormalities.
Case Conclusion:
CT head, maxillofacial, and cervical spine were obtained all of which were negative for acute traumatic findings with the exception of the lens dislocation which can be seen in Figure 2. The patient was observed in the emergency department until clinically sober and no further traumatic injuries were found on tertiary survey. Ophthalmology was consulted and recommended outpatient surgery for her traumatic lens dislocation.
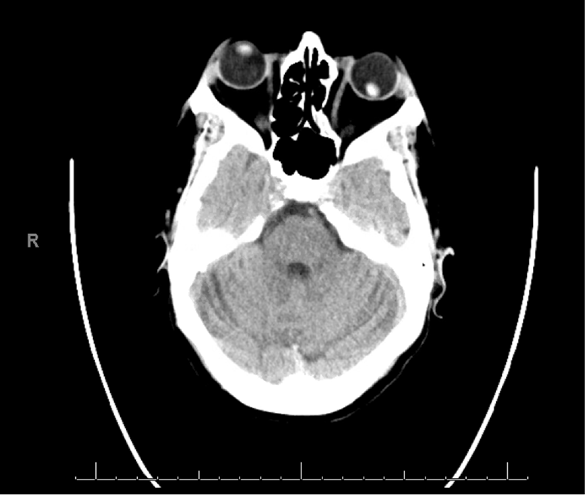
Figure 2: CT head image showing lens dislocation
Discussion:
Bedside ultrasound is an essential tool for rapidly identifying ocular pathology. Traumatic pathologies that can be seen on ocular ultrasound include globe rupture, vitreous hemorrhage, traumatic retinal detachment, lens dislocation, and foreign bodies. A linear transducer should be used with the machine set to an ocular setting or power manually reduced to at least 50%. A tegaderm can be used to protect the patient’s eye from gel, but be sure to remove all air from underneath it or image quality can be degraded. Scan the eyes in both the transverse and sagittal planes, and take care not to overgain your image or subtle findings can be missed.
Patients with lens dislocations often present with monocular diplopia. This is contrary to binocular diplopia which tends to have a neurological cause. Patients can present with complete anterior or posterior dislocations, or they can have subluxation of the lens. Anterior dislocations are emergencies as they can precipitate acute angle closure glaucoma by obstructing the aqueous outflow tract. Posterior dislocations and subluxations are managed more conservatively with many requiring non emergent outpatient surgery.
References
- Glickman A, Szczucki B, Kalivoda EJ, Furiato A, Cabrera G. Bedside Ocular Ultrasound Diagnosis of a Traumatic Lens Dislocation. Cureus. 2021 Apr 24;13(4):e14666. doi: 10.7759/cureus.14666. PMID: 33927958; PMCID: PMC8075821.
- Kilker BA, Holst JM, Hoffmann B. Bedside ocular ultrasound in the emergency department. Eur J Emerg Med. 2014 Aug;21(4):246-53. doi: 10.1097/MEJ.0000000000000070. PMID: 24002686.
- Wang HE, Ger DS, Gould SW. Diagnosis of traumatic lens dislocations. J Emerg Med. 2000 Jul;19(1):73-4. doi: 10.1016/s0736-4679(00)00183-9. PMID: 10950652.
Glimpse of Original Research
In this edition of the POCUS Report, we are featuring original ultrasound research abstracts, some which were presented at AAEM22.
Creating a Novel 3D-Printed Phantom to Simulate Ultrasound-Guided Pericardiocentesis
Louisa Weindruch, OMS-III, Taylor Terlizzese, OMS-III, Cassidy Miller, OMS-III
University of North Texas Health Science Center, Texas College of Osteopathic Medicine
Introduction:
With the growing impetus to provide increased patient safety, more programs are turning to simulation to teach invasive procedures to students. When learning ultrasound-guided procedures with high complication risk, such as pericardiocentesis, learners can use models called phantoms to mimic sonographic anatomy. Phantoms allow users to practice image acquisition and needle placement in a low-stakes environment. Unfortunately, procedural phantoms are extraordinarily expensive, making training with them often inaccessible to many learners. Homemade phantoms, while economically practical, tend to lack durability and anatomical accuracy. Our goal is to design an anatomically accurate phantom that most learners could make on their own for a relatively low price to practice ultrasound guided pericardiocentesis.
Methods:
To develop a new model, we investigated previous phantoms and other novel approaches to design an anatomically correct chest cavity. The product was created in a multistep process using the following materials:
Sternum:
To accurately simulate bone on sonography, the rib cage was engineered with computer-aided design and 3D-printed using polylactic acid through fused-deposition modeling with a 15mm thickness. The rib cage was then enclosed in a gelatin wax casing. A plastic container was used to hold the form of our model with a small bowl in the bottom to hold the place of the heart and pericardium.
Heart:
The heart was a styrofoam egg that was encased in a 12-inch latex balloon, to stimulate the pericardium. Water was then added to the balloon to stimulate the increased pericardial fluid in an effusion.
Skin:
The skin was made from vegetable-based glycerin, water, and gelatin. These were mixed in a 0.5 cup of glycerin to 0.5 cup water to 1oz gelatin (approximately 28 grams) ratio. Food coloring and cornstarch can be added for preferred color and consistency.
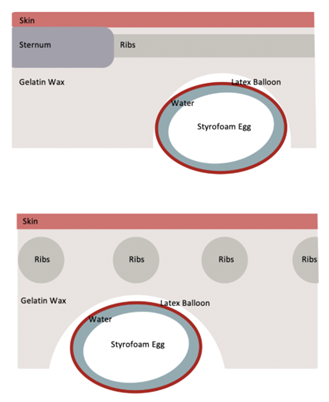
Figure 1: Two-view sketch of our final prototype
Methods & Results:
The development process included three prototypes, each with improvements to make the model more durable and anatomically correct.
Prototype 1:
The first prototype was made using a clay sternum, gelatin wax, and a latex theraband for skin. The clay ribs were molded and cooked in the oven prior to being encased in gelatin. There were several issues with this prototype. The ribs cracked and became disfigured when placed in the mold and the theraband skin did not stick to the sternum. When ultrasounding on this model, the skin came off and the gel wax began to fissure and come apart.
Prototype 2:
With this prototype, our focus was to improve the sternum and rib cage. We decided to proceed with a 3D-printed sternum. We found that this would still show rib shadowing and were hopeful that this would be a more solid structure than clay. We also created a liquid formula that could be poured on the gelatin to create a skin. The main issue with this prototype was that the gel wax was heated on the stove and therefore was a higher temperature than it needed to be. When this was poured into the mold, the heat unexpectedly deformed the 3D-printed ribs. The 3D-printed ribs also floated to the top of the gel wax. The deformation of the ribs made the spacing between the ribs non-anatomical and too small to ultrasound in between. The skin was an improvement and stuck to the model well while scanning.
Prototype 3:
Our final model was largely the same as prototype 2 in terms of materials. The main differences were that this 3D-printed model had a 15mm infill to help resist deformation secondary to heat and it was drilled into the plastic container to prevent floating. The gel was melted in the oven over several hours to obtain a uniform melt at a lower temperature. The same skin technique as prototype 2 was used.



Images 1, 2, 3: Left most is a top-down view of the final prototype. You can see the skin layer on top of the sternum and gelatin wax. Middle is the bottom of the final prototype that shows the ribs and the mold for the heart to sit in. Rightmost is a student practicing inserting a needle for pericardiocentesis.
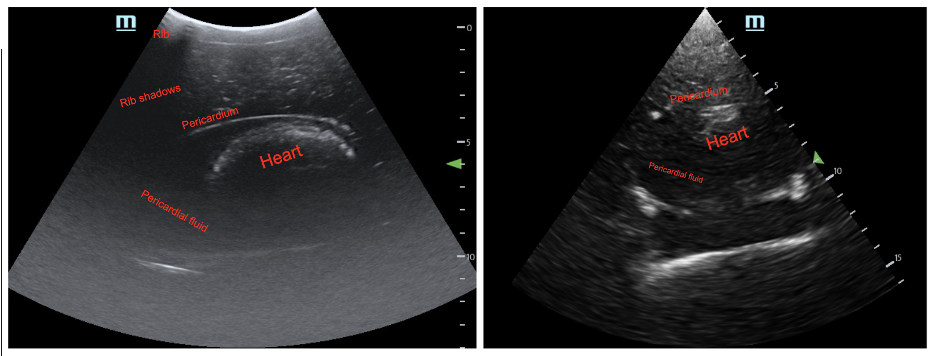
Image 4: Ultrasound image from the final prototype. Pediatric abdomen mode with curvilinear probe (left) and cardiac mode with phased-array probe (right).
Conclusion:
Ultimately, after much trial and error, prototype 3 was considered a success. We had a cohesive phantom without rib deformation that allowed us to obtain not only good ultrasound images, but to practice an ultrasound-guided pericardiocentesis. The total cost was also substantially less than that of a commercially made pericardiocentesis trainer. The final issue we are left with is that when practicing the pericardiocentesis, students can extract fluid, but ultimately the balloon that acts as the pericardium pops. The model as a whole is reusable, but in a mass practice session, each student would need their own balloon with a ‘pericardial effusion’ to insert into the model.
We hope this project can serve as a guide for other students who are interested in creating phantoms, both for pericardiocentesis or other procedures. Our difficulties reflect the many nuances of simulation, a growing field in medical education today. In the future, we plan to survey students and residents on their experience with our phantom. This will help us gain insight into user experience and ease of use.
References:
- Beaulieu A, Linden Az, Phillips J, Arroyo LG, Koenig J, et al. (2019) Various 3D printed materials mimic bone ultrasonographically: 3D printed models of the equine cervical articular process joints as a simulator for ultrasound guided intra-articular injections. PLOS ONE 14(8): e0220332. https://doi.org/10.1371/journal.pone.0220332
- Campo Dell'orto, M., Hempel, D., Starzetz, A., Seibel, A., Hannemann, U., Walcher, F., & Breitkreutz, R. (2013). Assessment of a low-cost ultrasound pericardiocentesis model. Emergency medicine international, 2013, 376415. https://doi.org/10.1155/2013/376415
- Daly, R., Planas, J. H., & Edens, M. A. (2017). Adapting Gel Wax into an Ultrasound-Guided Pericardiocentesis Model at Low Cost. The western journal of emergency medicine, 18(1), 114–116. https://doi.org/10.5811/westjem.2016.10.31506
- Young, T. P., & Kuntz, H. M. (2018). Modification of Daly's Do-it-yourself, Ultrasound-guided Pericardiocentesis Model for Added External Realism. The western journal of emergency medicine, 19(3), 465–466. https://doi.org/10.5811/westjem.2018.2.37736
- Zerth, H., Harwood, R., Tommaso, L., & Girzadas, D. V., Jr (2012). An inexpensive, easily constructed, reusable task trainer for simulating ultrasound-guided pericardiocentesis. The Journal of emergency medicine, 43(6), 1066–1069. https://doi.org/10.1016/j.jemermed.2011.05.066
Point of Care Ultrasound Diagnosis of Deep Vein Thrombosis by Medical Students
Cassidy Miller, OMS-3 and Sadie Thompson, OMS-3
Background:
Deep venous thrombosis (DVT) is the most common type of venous thromboembolism. The feared complication of a DVT is the progression to a pulmonary embolism. Many DVTs can resolve spontaneously, but screening and diagnosis are important for management to decrease progression to a pulmonary embolism and reduce the risk of morbidity and mortality. The purpose of this study was to determine if point-of-care ultrasound sonography by third-year medical students is sufficient in the diagnosis of a DVT.
Methods:
Fourteen students from the University of North Texas Health Science Center completed the relevant SonoSim training modules and a one-hour, in-person training session with an examination to determine that they have proficient skills to diagnose a DVT with a two-point ultrasound screening. Students then obtained images in the two-point DVT screening of the popliteal and femoral artery and vein on their rural family medicine rotation using Butterfly IQ Ultrasounds. Students saved images and videos of their screening and uploaded them to the Butterfly Cloud to be reviewed and graded. Studies will be deemed satisfactory if five out of six images obtained correctly identify the appropriate structures.
Results:
Of the fourteen third-year medical students that initially enrolled to be participants in this study, only eleven students completed the study. The eleven students that participated completed a total of 110 studies. Of these studies a total of 97 studies were deemed satisfactory after expert review, for an overall study satisfaction of 88.18%. Of the eleven students, seven participants had 100% satisfaction in their studies, one had 90%, one had 80%, one had 70% and one had 40%.
Conclusion:
We conclude that students are able to obtain satisfactory images of the deep legs of the vein using point of care sonography after a 1 hour training session. All students except for one who participated were able to demonstrate competency from the study images that were obtained and reviewed. Third year medical students can be proficient enough with point-of-care ultrasound to screen for DVT.
Discussion:
While students were able to obtain satisfactory images, the AAFP guidelines state that 25-50 scans should be done to determine general competency for a specific exam. In accordance with these guidelines, additional images would need to be obtained before students can be determined clinically competent. Additionally, we propose that students practice on a variety of standardized patients during future training sessions for exposure to patient related factors that could be secondary to patient participation, body habitus, and time with the patient. This proposition comes from the observations that students often performed really well on a study or missed all of the images for that study leading us to believe the ability to obtain satisfactory images was very patient based.
Utilizing 3-dimensional cardiac models with point of care ultrasound video tutorials to improve anatomic identification during medical student first time ultrasound exposure, a double-blinded randomized control study.
Hillary McKinley, MD, Heather Stuart, MD, Jordan Brunswick, MD, Shaina Ailawadi.
Wright State University Emergency Medicine Residency, Kettering, OH (HM, HS). Miami Valley Emergency Specialists, Dayton, OH (JB). Wright State University Boonshoft School of Medicine, Fairborn, OH (SA).
Objective:
This study aims to assess the role of three-dimensional (3d) cardiac models with point of care ultrasound (POCUS) cardiac tutorials to enhance cardiac ultrasound anatomic identification among medical students. The hypothesis was that the group who viewed the video tutorial with a 3d cardiac model would outperform the group that viewed the video without a 3d model. A subgroup analysis to assess ultrasound terminology and non-anatomic questions served as internal validation. To this extent, it was hypothesized that there would be no statistical significance between the two study arms in the subgroup analysis.
Methods:
A double-blinded, randomized control study was conducted among 39 volunteer first- and second- year medical students with no formal ultrasound training. The control group watched a video tutorial of cardiac anatomy using ultrasound imaging. The experimental group watched a similar video tutorial of cardiac anatomy using ultrasound imaging, but also had video references to a 3d cardiac model. The 3d model was a Learning Resources® Cross-Section Heart Model. The study subjects took a 24 question one-best-answer multiple choice test. Questions #1-8 (Q1-8) tested ultrasound principles while Questions #9-24 (Q9-24) tested cardiac anatomy. The reference images were printed on square paper in black and white, with color symbols used to label anatomic structures. No textual labels or image orientation was provided in an attempt to limit context clues.
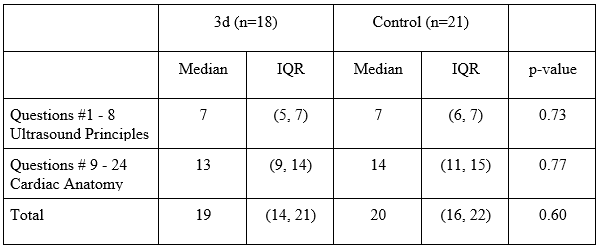
Result:
Q1-8 lacked normal distribution in each study arm, with a median of 7 for each study arm. The p-value of 0.73 reflected the lack of statistical significance between the two study arms for this subgroup, consistent with the initial hypothesis. Q9-24 again lacked normal distribution but had a median of 13 for the 3d arm and a median of 14 for the control arm. The p-value of 0.77 lacked statistical significance, not consistent with the initial hypothesis.
Conclusions:
Incorporating 3d cardiac models into video tutorials to improve medical student POCUS cardiac anatomy did not demonstrate statistical significance as compared to a control group without 3d models. However there were limitations within this study that may benefit from further study. The students in both arms scored higher than predicted which resulted in a severely underpowered study. Limiting the tutorials to a video presentation also mitigates the true effect of having a bedside 3d model, but did serve to ensure the uniformity of pre-test tutorials of either study arm. This study utilized an innovative testing method that holds promise for future research. As far as the authors are aware, this is the first study to utilize 3d cardiac models within ultrasound education.
Acknowledgements:
The authors wish to thank Dr. Catherine Marco, MD for her guidance and patience during the research process as well as Nancy Bruderer, MS, for her statistical expertise and assistance with data analysis. The authors would also like to thank Dr. Andrea Kaelin for her ultrasound skill, technical expertise, and mentorship during residency.
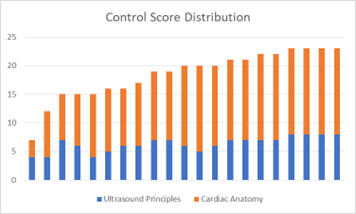
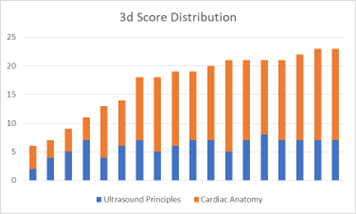
Figure 1: Score distribution of control study arm (left) and 3d study arm (right).
Figure 2: Summary of test scores.
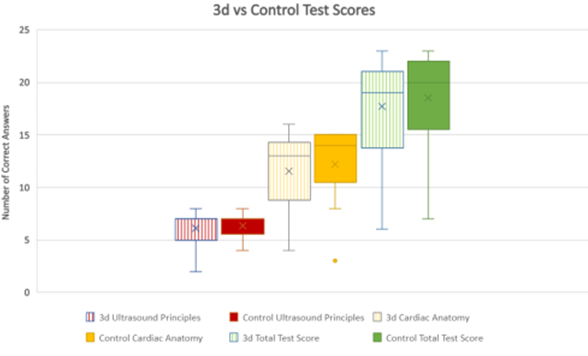
Figure 3: Box and whisker plot of test scores.