Summer 2021
Welcome from EUS-AAEM
The Emergency Ultrasound Section of the American Academy of Emergency Medicine (EUS-AAEM) is founded to foster the professional development of its members and to educate them regarding point-of-care ultrasound. This group will serve as a venue for collaboration among medical students, residents and practitioners who are interested in point-of-care ultrasound. The purpose of our group is to augment the knowledge and expertise of all emergency medicine specialists and to advocate for patient safety and quality care by endorsing bedside ultrasound. Membership is not limited to fellowship trained physicians. All emergency medicine practitioners passionate about ultrasound are welcome to join and participate.
We are proud to publish our e-newsletter with original contributions from many of our members. We encourage all members to submit for future editions. Topics include but are not limited to educational, community focus, interesting cases, resident and student section, and adventures abroad.
For more information visit us online at: www.aaem.org/EUS.
In this Issue:
-
President's Message
-
Ultrasound Building Blocks: Pathologic Sonographic Findings in Acute Appendicitis
-
Resident Section: Point-of-Care Ultrasound (POCUS) Findings in Necrotizing Soft Tissue Infection
-
Resident Section: Chamber Collapse in Pericardial Effusion
-
Clinical Images: POCUS for Rapid Diagnosis of Patellar Fracture
-
Clinical Images: Don’t be Fooled, It’s just the Bladder
-
Fellow Section: Early Identification of Thoracic Aortic Dissection with Suprasternal Notch View
-
Ultrasound Guided Procedures: Ultrasound Guided Popliteal Sciatic Nerve Block for a Complex Laceration on the Anterior Shin: A Case Report
-
Ultrasound Guided Procedures: Ultrasound Guided Brachial Plexus Nerve Block via The Retroclavicular Approach to the Infraclavicular Region (RAPTIR)
-
Ultrasound Saves: Musculoskeletal Ultrasound guided needle aspiration of a Septic Subdeltoid Bursitis
-
Ultrasound Saves: Evaluation for Bladder Rupture
Chairs's Message
Members of the EUS-AAEM Section,
I am honored to have the opportunity to serve as your EUS-AAEM Council Chair this year. This is an exciting time for the Emergency Ultrasound Section. We just gathered in person for the first time in over a year at the hybrid 27th AAEM Scientific Assembly in St. Louis.
As EUS Council Chair, I intend to focus on our continued commitment to physicians practicing in both community and academic environments. We will work to provide resources to community physicians working to incorporate ultrasound into their practice and programs preparing for accreditation through the Emergency Ultrasound Fellowship Accreditation Council. Our section will continue to have an impact through the continuation of the successful “Unmute Your Probe” lecture series, in-person teaching at the Scientific Assembly, and the continuation of the skills verification workshops.
I look forward to working with all of you over the next year as we work together to improve patient care with emergency ultrasound.
Melissa Myers, MD FAAEM
EUS-AAEM President, 2021-2022
Ultrasound Building Blocks
Pathologic Sonographic Findings in Acute Appendicitis
Alexis Salerno, MD FAAEM
Introduction
Studies have shown that there is greater patient satisfaction with using bedside ultrasound in the evaluation of a patient.1 As bedside ultrasound becomes prominent, there have been multiple articles describing the use of point of care ultrasound in diagnosing acute appendicitis at bedside.2-4 These articles conclude that in a skilled operator and in a population with a high pretest probability, bedside ultrasound is a useful tool in diagnosing acute appendicitis especially in the pediatric population.3 There are many different pathological findings on ultrasound that can suggest acute appendicitis in a patient. In this article we will go over five pathologic sonographic findings in acute appendicitis.
How to Scan
To scan the appendix, you can use the high frequency linear probe or curvilinear probe depending on your patient’s BMI. While the patient is lying supine, I have found it helpful to ask the patient to point with one finger to the area where the pain is most severe. You can then place the transducer transversely over the area. If the patient is unable to do this, you can scan a larger area of the right lower quadrant in an organized fashion. You can identify the appendix laying over the psoas muscle and it will appear as a noncompressible tubular structure with no peristalsis or flow. Once the appendix is identified, scan the structure in both the short and long axis. In the short axis view, measure the diameter from outer wall to outer wall.
Pathologic Findings:
- Target Sign
As the appendix becomes inflamed, a thickened edematous appendix can be identified on ultrasound. The literature also describes a “target sign” appearance of the appendix on the short axis. This is due to hypoechoic fluid at the center of the appendix surrounded by a hyperechoic submucosal layer and an outer hypoechoic ring.5 Appendicitis is highly unlikely if the wall diameter is less than 6 cm.
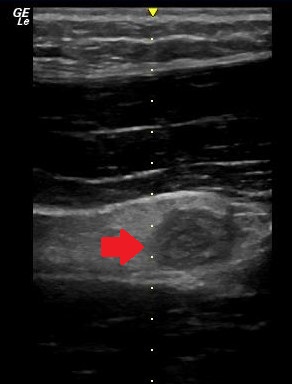
Figure 1. Transverse view of an inflamed appendix demonstrating the target sign (arrow).
- Appendicolith
Some cases of acute appendicitis are thought to be caused by obstruction of the appendix by an appendicolith. On ultrasound, appendicolith can be identified as a hyperechoic structure which casts a posterior acoustic shadow.5
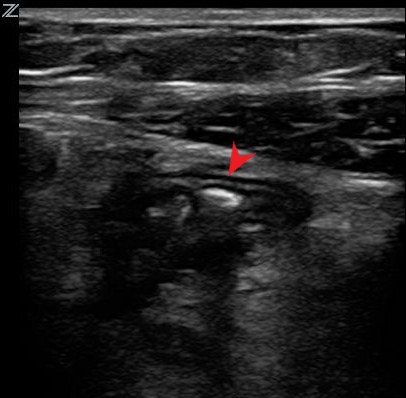
Figure 2. Long axis view of an appendix with an appendicolith.
- Evidence of Inflammation
The process of acute appendicitis represents an active inflammatory process. Therefore, on ultrasound physicians will be able to appreciate periappendiceal fluid, dilated loops of bowel and enlarged inflamed lymph nodes. In addition, increased echogenicity and color flow may be noticed surrounding the appendix which correlates to fat-stranding noticed on CT.5
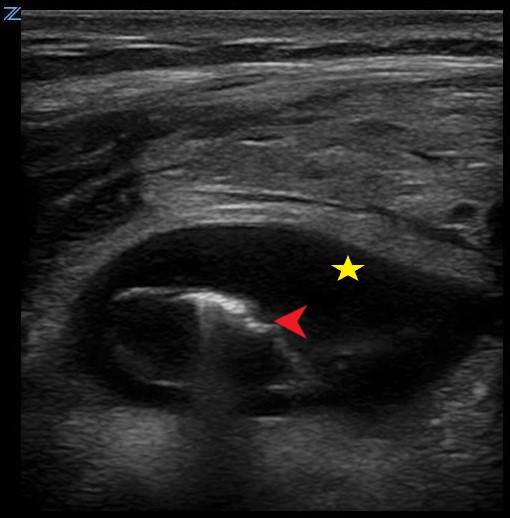
Figure 3. Appendicitis demonstrating periappendiceal fluid (star) and reverberation artifact from free air (arrow).
- Perforation
In some patients, such as older individuals and diabetics, there may be a delay to diagnosis. These patients are at higher risk for perforation of the appendix. On ultrasound, free air can be identified as a reverberation artifact (Figure 3, Figure 4). As the disease advances you may see adjacent debris suggestive of a phlegmon or an abscess. In addition, physicians can see loss of the echogenic submucosal layer which suggests gangrene of the appendix.5,6
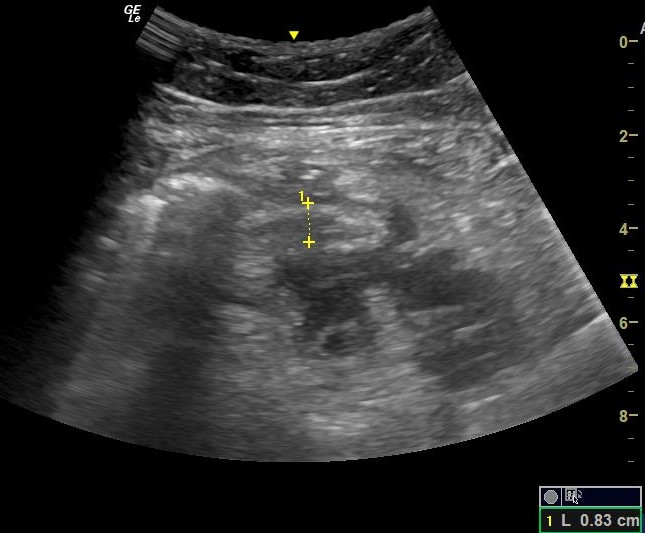
Figure 4. Perforated appendicitis. Phlegmon formation noted posterior to the appendix.
- Increased Renal Echogenicity
Normally the kidney has slightly lower echogenicity as compared to the liver. Recent studies in children have suggested that in acute appendicitis the right kidney may display increased echogenicity as compared to the liver. In a study by Tal Tamir et al. the renal cortex had increased echogenicity in 49.9% of patients who were diagnosed with acute appendicitis. More studies are needed in adults to confirm this association.7
References
- Howard ZD, Noble VE, et al. Bedside ultrasound maximizes patient satisfaction. J Emerg Med. 2014 Jan;46(1):46-53. doi: 10.1016/j.jemermed.2013.05.044. Epub 2013 Aug 12.
- Corson-Knowles D, Russell FM. Clinical Ultrasound Is Safe and Highly Specific for Acute Appendicitis in Moderate to High Pre-test Probability Patients. Western Journal of Emergency Medicine. 2018;19(3):460-464. doi:10.5811/westjem.2018.1.36891.
- Lee SH, Yun SJ. Diagnostic performance of emergency physician-performed point-of-care ultrasonography for acute appendicitis: A meta-analysis. Am J Emerg Med. 2018 Jul 14. pii: S0735-6757(18)30582-5. doi: 10.1016/j.ajem.2018.07.025.
- Matthew Fields J, Davis J, et al. Accuracy of Point-of-care Ultrasonography for Diagnosing Acute Appendicitis: A Systematic Review and Meta-analysis. Acad Emerg Med. 2017;24(9):1124-1136.doi: 10.1111/acem.13212.
- Quigley AJ, Stafrace S. Ultrasound assessment of acute appendicitis in paediatric patients: methodology and pictorial overview of findings seen. Insights into Imaging. 2013;4(6):741-751. doi:10.1007/s13244-013-0275-3
- Xu, Y. , Jeffrey, R. B., Chang, S. T., et al. (2017), Sonographic Differentiation of Complicated From Uncomplicated Appendicitis: Implications for Antibioticsâ€First Therapy. J Ultrasound Med, 36: 269-277. doi:10.7863/ultra.16.03109
- Tal Tamir H, Ben-Mordechay D, et al.Increased Renal Echogenicity in Children With Appendicitis. J Ultrasound Med. 2018 Jun;37(6):1403-1409. doi: 10.1002/jum.14480.
Resident Section
Point-of-Care Ultrasound (POCUS) Findings in Necrotizing Soft Tissue Infection
Natalie Truong, MD and Jason Young, MD
Introduction
Necrotizing fasciitis is a soft tissue infection associated with high morbidity and mortality. Accurate diagnosis and appropriate treatment are necessary.
Sonography can aid in early diagnosis of necrotizing fasciitis if clinical suspicion is high.
Case Presentation
A 49-year-old woman with a past medical history of hypertension, hyperlipidemia and uncontrolled type 2 diabetes mellitus, complicated by first and second left toe amputation and non-healing right foot ulcer s/p debridement who presented to the emergency department with a 5-day history of progressively worsening left lower extremity edema and blisters on the left ankle. On review of symptoms, the patient did not have associated fever, chills, night sweats, or proceeding trauma.
On examination, she was febrile 103.3°F, heart rate 104 bpm, blood pressure 109/59, respiratory rate 20 bpm, and oxygen saturation of 100% on room air. She was in no distress and appeared well. The integumentary exam of the left lower extremity showed poorly demarcated erythema with maroon-colored discoloration distally, with associated warmth and bulla over the left lateral malleolus. There was 2+pitting edema with pain and crepitus along the left dorsal lateral malleolus extending towards the tibia plateau. The left popliteal pulse was palpable.
Ultrasound was performed using a (13-6 MHz) linear probe. Point of Care Ultrasound (POCUS) of left lower extremity showed increased echogenicity of the overlying subcutaneous fat, thickening of the fascia, hyperechoic soft tissue emphysema with posterior “dirty” acoustic shadowing, and a deep perifascial fluid collection. All of the above signs were highly suggestive of necrotizing soft tissue infection.
 Figure 1 |
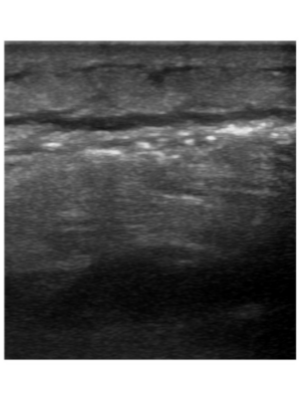 Figure 2 |
Transverse view of left anterior-lateral leg on bedside point-of-care ultrasound demonstrates a thin anechoic collection of fluid, layered deep or posterior to edematous soft tissue with marked “cobblestoning” appearance. This finding represents perifascial fluid spreading anteriorly across the deep fascial plane.
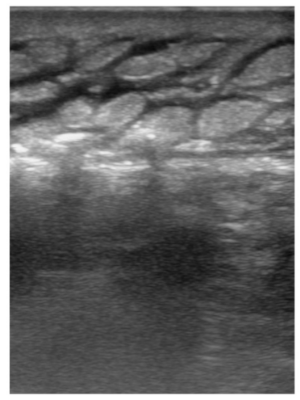
Figure 3
Transverse view of left leg with visualization of deep fascial line as depicted by a linear, horizontal, stripe, with increased variable, thickness, and a frequent, irregular, break, pattern.
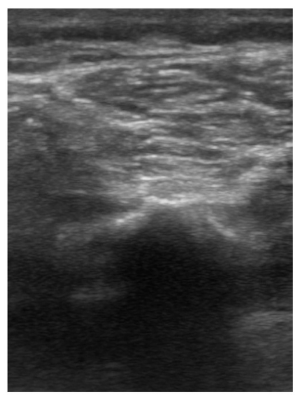
Figure 4
Comparison view of right anterior-lateral leg with visualization of normal appearing fascial line depicted as thin, homogeneous, hyperechoic, stripe.
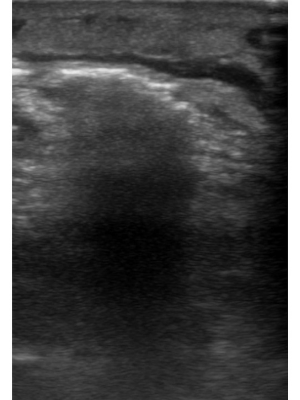
Figure 5
Heterogeneous, non uniform, or “dirty,” posterior acoustic shadowing is seen emanating from the echogenic line, representative of gas formation.
Radiograph of the left foot, ankle and tibia-fibula showed diffuse subcutaneous emphysema extending along the lateral aspect of the left lower extremity from the knee down to the ankle consistent with necrotizing fasciitis. There was also diffuse acute osteomyelitis of the left foot involving the amputation margins of the first and second metatarsals, third metatarsal and proximal phalanx.
The patient’s LRINEC score was 10. Patient was taken to the operating room and underwent emergent left guillotine below knee amputation and debridement of necrotic tissue. The patient tolerated the procedure well and transferred to the post anesthesia care unit in stable condition. The patient was found to have gram-positive bacteremia with positive blood cultures for Parvimonas micra, formally Peptostreptococcus.
Discussion
The 49-year-old woman depicted in this case had multiple risk factors for the development of a necrotizing soft tissue infection (NSTI). Considering her history of obesity, uncontrolled diabetes with past complications of osteomyelitis, metabolic syndrome, and poor wound healing, she essentially had an impaired immune response with an undiagnosed peripheral vascular disease, and a resultant increased susceptibility to infection of the distal extremities.1 Given her immunocompromised state, any minor breach in skin barrier could have easily resulted in introduction of infection and rapid spread.2,3 The organisms isolated from the patient's blood cultures are consistent with the polymicrobial type I, or “mixed,” classification of NSTI, which contains anaerobic bacteria.4 Presumptively, this patient may have suffered minor penetration to the foot, with rapid spread to the fascial compartments and ascension up the leg. Consequently, the variable and vague clinical presentation of NSTI, lends it to high rates of misdiagnosis and mortality. Therefore, it is important to recognize clinical cues, and avoid false attribution of patient signs and symptoms to other causes.
Even in the absence of cutaneous findings, the hallmark findings of “pain out of proportion to exam,” or systemic manifestations (i.e. tachycardia, hypotension, elevated CRP, elevated CK levels) within the proper clinical context warrants prompt imaging and early consideration of NSTI. Although x-ray is typically limited in its application in most cases, this patient had a pathognomonic finding of subcutaneous emphysema as seen on a plain film of her left lower extremity. This is caused by Clostridium species, gas-forming NSTIs, and rare occurrences of other NSTI types. In the absence of subcutaneous emphysema, CT and MRI also have a role in imaging of NSTI with deep tissue changes of fascial thickening, fluid collection, and contrast enhancement, but the gold standard diagnosis remains surgical exploration. Compared to MRI, ultrasonography is preferred in patients who are hemodynamically unstable, have contraindications to MRI, have renal insufficiency or contrast allergy and specifically, it shows higher resolution, immediate results, and cost effectiveness.5 Furthermore, with gas formation in the tissues being a late sign of infection, ultrasound offers the benefit of earlier recognition of NSTI through multiple findings seen in both early and late stages of infection.
Conclusion
Necrotizing fasciitis typically spreads along the muscle fascia and overlying subcutaneous fat due to their relatively poor blood supply and the superficial tissue can initially appear normal making diagnosis difficult. Point-of-care ultrasound is utilized to evaluate characteristic findings such as asymmetric fascial thickening, poorly defined fascia, perifascial fluid, and echogenic foci of air within the deep tissue. Hyperechogenic soft tissue emphysema with posterior “dirty” acoustic shadowing, and perifascial fluid collection tracking along the deep fascia strongly suggests necrotizing fasciitis rather than a more superficial skin infection.
References
- Goh T, Goh L.G., Ang C.H., et al. Early diagnosis of necrotizing fasciitis. Br J Surg. 2014 Jan; 101(1):e119-25. DOI: 10.1002/bjs.9371. Epub 2013 Nov 29. PMID: 24338771.
- Hirji, S., Taghavi, S., and Askari, R. (2019). Necrotizing Soft Tissue Infection: A Practical Approach. DOI: 10.1007/978-3-319-96286-3_38.
- Stevens, D.L. and Bryant, A.E. Necrotizing Soft-Tissue Infections. N Engl J Med. 2017 Dec 7; 377(23): 2253-2265. DOI: 10.1056/NEJMra1600673. PMID: 29211672.
- Hakkarainen, T.W., Kopari, N.M., Pham, T.N., et al. (2014). Necrotizing soft tissue infections: review and current concepts in treatment, systems of care, and outcomes. Current problems in surgery, 51(8), 344–362. DOI: 10.1067/j.cpsurg.2014.06.001
- Gottlieb, J., Mailhot, T., and Chilstrom, M. Point-of-care ultrasound diagnosis of deep space hand infection. The Journal of Emergency Medicine. 2016; 50(3): 458-461. DOI: 10.1016/j.jemermed.2015.09.012
Chamber Collapse in Pericardial Effusion
Anisa Mughal, MD and Levi Filler, DO
Case Presentation
A 39-year-old transgender female with a past medical history of breast augmentation and unmedicated diabetes mellitus type II presented to the emergency department with one week of bilateral peripheral leg swelling. She complained of orthopnea, paroxysmal nocturnal dyspnea and dyspnea on exertion. She denied chest pain, recent viral infections, fever/chills and illicit substance use.
Initial vitals were notable for a T 36.5C, P 102, BP of 186/126, RR 16, SpO2 100% on RA, . She appeared comfortable and in no acute distress on exam. She had 2+ pitting edema below the knees bilaterally with no overlying erythema, lesions, or rashes. No appreciable cardiac murmurs, rubs, or gallops were present on cardiopulmonary exam, but there were trace crackles upon lung auscultation.
Labs revealed an elevated BNP, negative troponin, elevated creatinine, negative UDS, and a negative COVID test. Chest x-ray revealed bilateral pleural effusions.
A bedside cardiac echo was performed by the treating physician and revealed reduced LV contractility as well as a pericardial effusion with evidence of right atrial collapse. The right ventricle was evaluated as well with questionable collapse in diastole. She remained hypertensive in the 180’s/110’s and tachycardic. A nitroglycerine drip was initiated, and she was subsequently transferred to the medical ICU. Formal cardiac echo upon admission revealed a mildly reduced EF of 41-53% with grade II diastolic dysfunction, mild left atrial enlargement, mild mitral regurgitation, mild pulmonary hypertension, and moderate pericardial effusion. No obvious tamponade physiology was appreciated on the formal echocardiogram.
Figure 1. Right atrial collapse in the setting of pericardial effusion on bedside cardiac echo
Discussion
There are multiple etiologies of pericardial effusions, including trauma, infection, post-surgical changes, and clotting abnormalities. Pericardial tamponade is a feared complication of pericardial effusions and can be diagnosed both clinically and sonographically. Classic clinical findings of tamponade include JVD, hypotension, and muffled heart sounds.2 Although historically thought of as a clinical diagnosis, there are several considerations to make when considering cardiac tamponade in subacute presentations. It is estimated that up to 43% of tamponade patients may present with hypertension, especially in the setting of renal dysfunction.1 Symptoms such as dyspnea, shortness of breath and JVD lack both sensitivity and specificity and must be considered along with vital signs and clinical signs and symptoms.3 With the advent of bedside point-of-care ultrasound (POCUS), a more accurate evaluation of tamponade can be achieved.5 Echocardiography has been a useful imaging tool to assess the kinetics of the heart, pericardial effusion and hemodynamic compromise. With tamponade physiology, the right atrium (RA) is the first to collapse, followed by the right ventricle (RV).2 M-mode echocardiography can be used to identify right sided ventricular wall collapse in diastole by recording motion over time. This is particularly useful when performing bedside POCUS, as up to one third of patients without clinical features of cardiac tamponade may have at least one chamber collapse on echocardiography.3 The sensitivity and specificity vary for RA and RV collapse indicating cardiac tamponade vary from 48% to 100%;4 transient right atrial collapse is commonly seen but is not specific.2,3 The duration of atrial collapse (more than one third of the cardiac cycle) has been described as nearing 100% sensitivity and specificity for tamponade.2,4,5 Collapse of both atria increases sensitivity and specificity for tamponade, however these findings may not be present in patients with pulmonary hypertension or increased ventricular diastolic filling pressures.2
Limitations of echocardiography include interpretation of effusion size and chamber collapse.3 Rate of accumulation of pericardial fluid is a more accurate predictor of patients developing tamponade and is not well-assessed by POCUS2. Furthermore, the angle of the probe may overestimate the size of the effusion.2 Additionally, respiratory flow variation and IVC collapsibility can be a useful adjunct to assess for increased right-sided filling pressures.2,3
Our patient had transient atrial collapse in the setting of pericardial effusion and hypertension as seen on POCUS. She was subsequently admitted for a formal echocardiogram which did not reveal a diagnosis of cardiac tamponade. Given these findings, providers should be judicious when caring for patients with findings of right atrial collapse in the setting of a pericardial effusion, but no clinical signs consistent with tamponade. Bedside POCUS and accurate image interpretation may help avoid unnecessary procedural interventions as well as to diagnose early signs of pericardial tamponade.
Conclusion
Bedside POCUS can be a useful tool when assessing those with atypical features of pericardial tamponade. Providers should recognize that findings of right atrial collapse in the setting of a pericardial effusion is non-specific, but may represent early pericardial tamponade. Accurate interpretation of POCUS results along with the patient’s clinical presentation can assist providers in determining the best possible course of care for their patients.
References
- Argulian E, Herzog E, Halpern DG, et al. Paradoxical hypertension with cardiac tamponade. Am J Cardiol. 2012 Oct 1;110(7):1066-9. doi: 10.1016/j.amjcard.2012.05.042.
- Pérez-Casares A, Cesar S, Brunet-Garcia L, et al. Echocardiographic Evaluation of Pericardial Effusion and Cardiac Tamponade. Front Pediatr. 2017;5:79. doi:10.3389/fped.2017.00079
- Argulian E, Messerli F. Misconceptions and facts about pericardial effusion and tamponade. Am J Med. 2013 Oct;126(10):858-61. doi: 10.1016/j.amjmed.2013.03.022.
- Guntheroth WG. Sensitivity and specificity of echocardiographic evidence of tamponade: implications for ventricular interdependence and pulsus paradoxus. Pediatr Cardiol. 2007 Sep-Oct;28(5):358-62. doi: 10.1007/s00246-005-0807-9.
- Singh S, Wann LS, Schuchard GH, et al. Right ventricular and right atrial collapse in patients with cardiac tamponade--a combined echocardiographic and hemodynamic study. Circulation. 1984 Dec;70(6):966-71. doi: 10.1161/01.cir.70.6.966.
Clinical Images
POCUS for Rapid Diagnosis of Patellar Fracture
Shawn Sethi, DO
PGY-4 Emergency Medicine
Einstein Healthcare Network
A 54-year-old female presented to the emergency department via EMS after she sustained a trip and fall onto her ride side. She was experiencing severe pain to her right leg that was rated as 10/10. The patient was unable to ambulate after the fall due to pain and noticed swelling to her right knee.
Her physical exam was notable for a visible and palpable effusion to the inferior aspect of the right knee along with significant tenderness to palpation to the right patella. Additionally, the patient had a deformity to her right patella. The patient did not have associated erythema, warmth or breaks in the skin. She was unable to flex or extend at the knee secondary to pain but did have strong DP and PT pulses with intact light touch sensation to her right leg.
Prior to obtaining x-rays, bedside ultrasound was used to investigate the patient’s right knee bony deformity. A linear probe with a large amount of gel was used in order to properly image the superficial structures, while avoiding excessive contact with the knee. A sagittal image of the patient’s knee is presented below, with labeled structures.
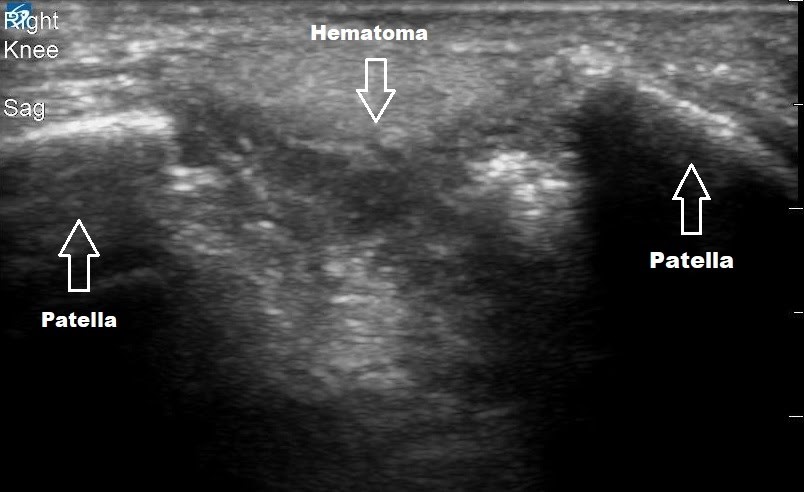
Figure 1. Sagittal view of the right knee using a linear high frequency probe
In this image, the fractured patella is seen with two distinct hyperechoic segments on either side of a hypoechoic hematoma and surrounding soft tissue edema. Not pictured are ultrasound images of the intact quadriceps and patellar tendon, just superior and inferior respectively to the image shown. Note the correlation with the patient’s knee x-ray which was taken afterwards.
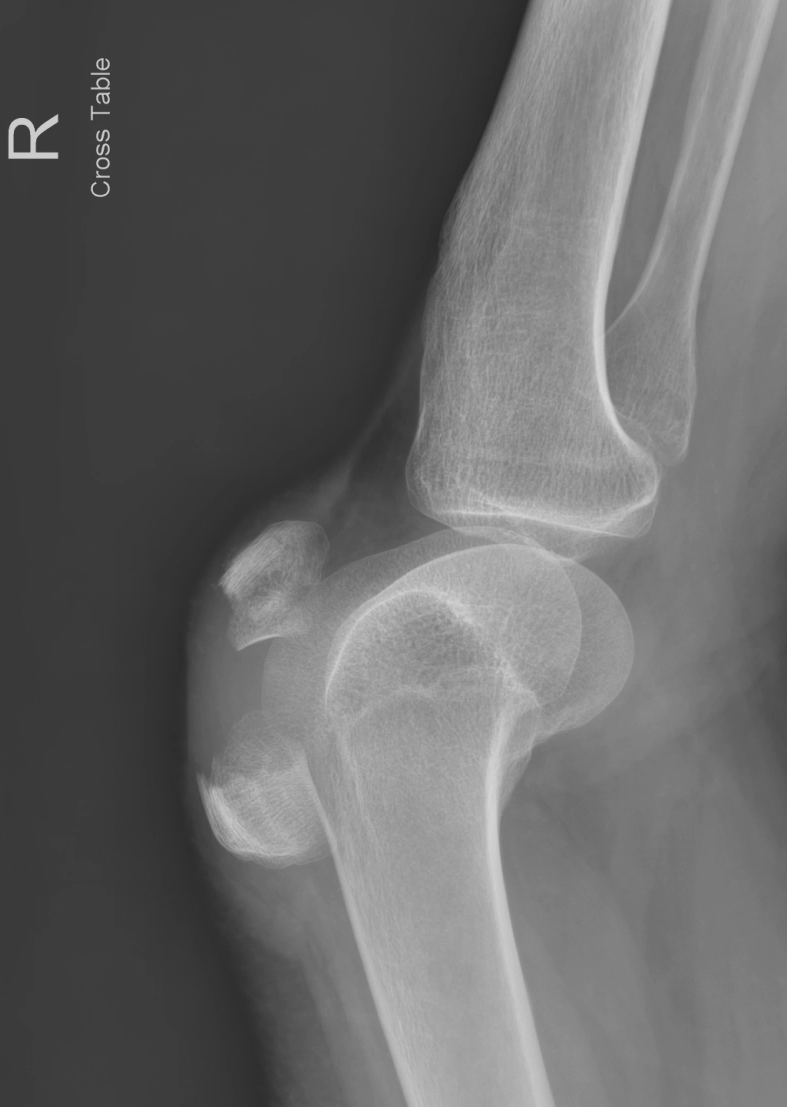
Figure 2. Lateral radiograph of the right knee
Although x-ray remains the most commonly used modality, there are several advantages with point of care ultrasound for imaging musculoskeletal structures in the emergency department. X-ray produces ionizing radiation, requires large portable machines or transportation to a radiology suite, specialized imaging software to view images and can lead to diagnostic delays in a busy emergency department. On the contrary, point of care ultrasound provides a portable, dynamic and safe imaging modality for those with musculoskeletal complaints. This is especially true in resource limited settings where access to x-ray or CT may be difficult.
The body of evidence for use of ultrasound for bony injuries continues to grow. Several studies have found accuracy of POCUS for ankle and metacarpal fractures as high as 92% sensitive and 87% specific.1-2 Additionally, bedside ultrasound may be helpful in detecting lipohemarthrosis, the layering of fat and blood. This finding is more suggestive of intra-articular fracture than joint effusion, with an accuracy higher than x-ray.3 Finally, ultrasound can be helpful in diagnosis of tendon injury. A prospective study of 34 patients from the American Journal of Emergency Medicine found bedside ultrasound 97% accurate for identifying upper and lower extremity tendon injuries and was approximately 92 minutes faster than surgical exploration or MRI.4
The diagnosis and disposition for this patient was rapidly made after POCUS, prior to any X-ray studies. On your next shift in the ED, consider using ultrasound to assist with diagnosis of fracture or tendon injury in patients with musculoskeletal complaints.
References
- Atilla OD, Yesilaras M, Kilic TY, et al. The accuracy of bedside ultrasonography as a diagnostic tool for fractures in the ankle and foot. Acad Emerg Med. 2014 Sep;21(9):1058-61. doi: 10.1111/acem.12467.
- Kozaci N, Ay MO, Akcimen M, et al. The effectiveness of bedside point-of-care ultrasonography in the diagnosis and management of metacarpal fractures. Am J Emerg Med. 2015 Oct;33(10):1468-72. doi: 10.1016/j.ajem.2015.06.052.
- Bonnefoy O, Diris B, Moinard M, et al. Acute knee trauma: role of ultrasound. Eur Radiol. 2006 Nov;16(11):2542-8. doi: 10.1007/s00330-006-0319-x.
- Wu TS, Roque PJ, Green J, et al. Bedside ultrasound evaluation of tendon injuries. Am J Emerg Med. 2012 Oct;30(8):1617-21. doi: 10.1016/j.ajem.2011.11.004.
Don’t be Fooled, It’s Just the Bladder
Jessica Downing, MD
Case Presentation
A 52-year-old male presented to the ED after calling EMS for abdominal pain for two days, accompanied by nausea and vomiting. On EMS arrival to the patient’s home, he reported feeling lightheaded and being unable to stand due to his lightheadedness. A mental status exam was normal. Vital signs were notable for a blood pressure of 53/32 and were otherwise normal. Repeat blood pressure after transport was unchanged. He denied any traumatic injury. The only medical history he could provide was a history of HIV. Other vital signs, including temperature, were normal. His physical exam was notable only for a protuberance of the lower abdomen, extending just above the umbilicus, with diffuse but mild abdominal tenderness to palpation. A small scar was noted approximately halfway between the pubic symphysis and the umbilicus, which the patient reported was the site of a prior suprapubic catheter.
Point of Care Ultrasound (POCUS) was immediately obtained with the initial goal of assessing for an Abdominal Aortic Aneurysm (AAA). The aorta was found to be normal in caliber with no aneurysmal dilatation. However, a complex fluid-filled structure was noted superficial to the aorta, extending from the pelvis superior to the umbilicus (Figure 1). This was noted to be superficial to a structure more consistent in location and shape with the urinary bladder; although the two structures were separated by a wall, there was a communication between the two, with free flow of urine observed with color Doppler, leading to concern for bladder rupture and urinoma collection versus complex bladder diverticula. POCUS renal ultrasound demonstrated bilateral moderate hydronephrosis. A Foley catheter was placed and was identified within the superficial fluid collection, further suggesting the presence of diverticula.
Placement of the Foley was followed by immediate drainage of 1.2 L of clear urine. The patient’s blood pressure improved with IV fluids and decompression of the bladder. On further questioning, the patient reported acute urinary retention for two days. He reported a history of prior similar events.
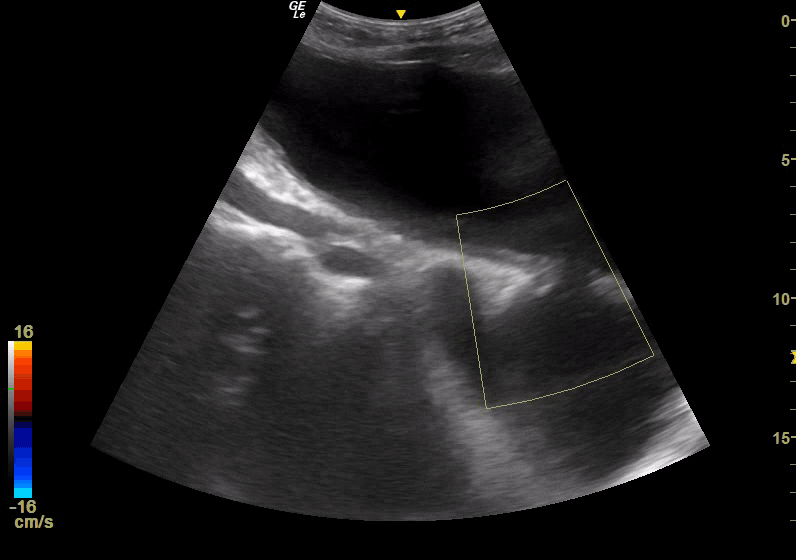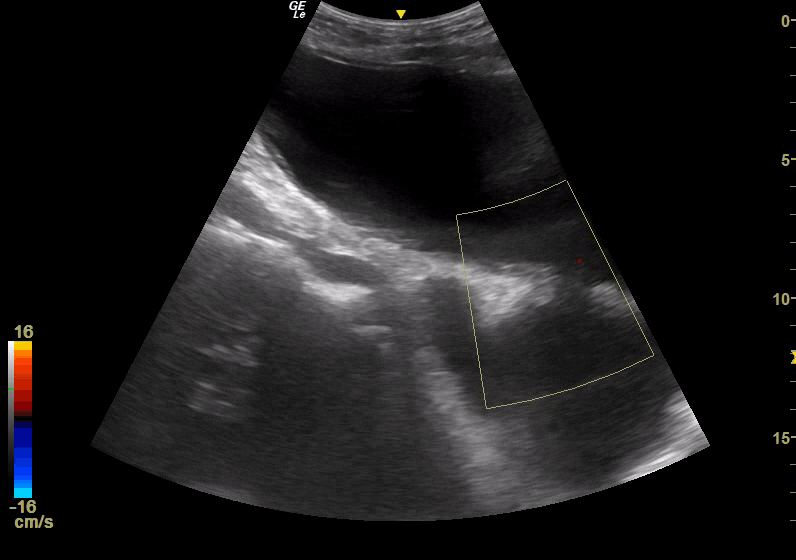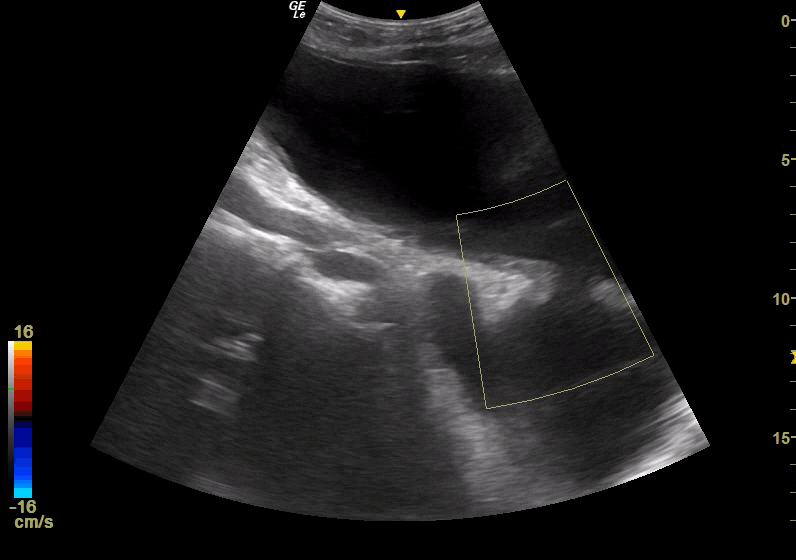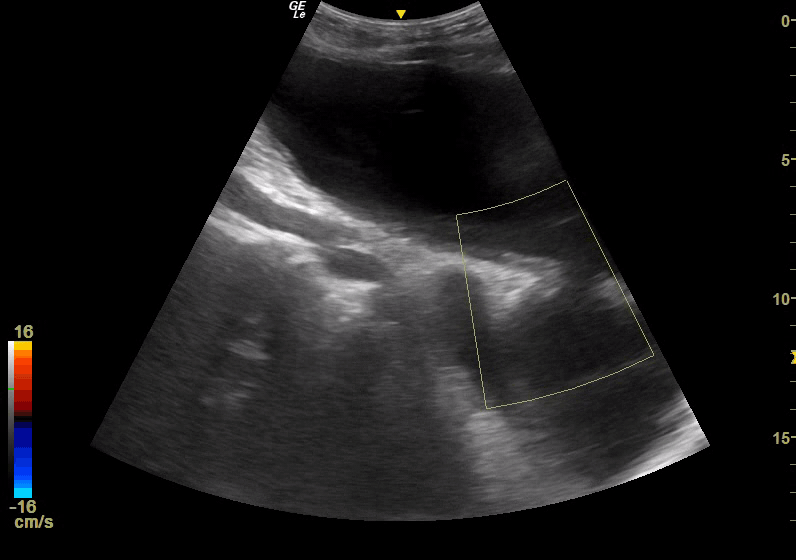
Figure 1
Case Conclusion
Review of the patient’s prior imaging demonstrated known bladder diverticula and chronically thickened bladder walls, though hydronephrosis had not been previously identified. This was confirmed on a CT obtained during this Emergency Department visit. The patient’s condition stabilized during Emergency Department observation, and he was ultimately admitted to the medicine floor with urology consult and anticipated replacement of a suprapubic catheter.
Learning Points
On ultrasound, bladder diverticula can be identified by locating a communication between the bladder and a cystic mass.1 By using color flow doppler, you can demonstrate flow between the cystic mass and the bladder. Urinomas are also encapsulated, but can appear anywhere along the urinary tract where there is urine leakage. In a patient with a bladder rupture, you are less likely to see an encapsulated area and may see free intraperitoneal fluid. On ultrasound, it may be difficult to differentiate a urinoma or a bladder rupture from a bladder diverticulum. Therefore, further imaging such as a CT scan are recommended.
References
- Maynor CH, Kliewer MA, et al. Urinary bladder diverticula: sonographic diagnosis and interpretive pitfalls. J Ultrasound Med. 1996;15(3):189-94. doi: 10.7863/jum.1996.15.3.189.
Fellow Section
Early Identification of Thoracic Aortic Dissection with Suprasternal Notch View
Richard Cunningham, MD and Levi Filler, DO
Case Presentation
A 47-year-old male with a history of aortic insufficiency and congestive heart failure secondary to cocaine use presented to the emergency department with intermittent back pain. His pain had been present for two days and was located along the midline lower back with radiation to the upper back. There was no history of injury or trauma. He presented to the Emergency Department (ED) because he awoke with 10/10 pain and diaphoresis at approximately 0400 the night prior, however, upon initial presentation, he rated his pain at 3/10. His vitals were: T 36.8°C, P 88 bpm, BP 149/71 mmHg, RR 16 and SpO2 97%. His exam revealed a well-appearing male in no acute distress with full range of motion of the back and no midline or paraspinal tenderness. Cardiac exam revealed a prominent diastolic aortic regurgitation murmur which had been noted on previous visits in the outpatient setting on chart review. Given the patient's history of aortic insufficiency, there was concern for aortic pathology as a cause of his back pain. A point-of-care ultrasound (POCUS) was performed which revealed the following images:
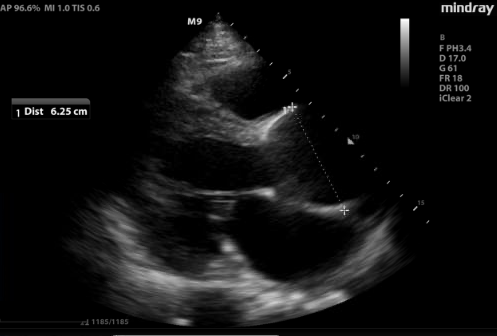
Figure 1. Parasternal long view of the heart revealed a significantly dilated aortic root (6.25 cm), representing increased risk of developing acute aortic pathology.
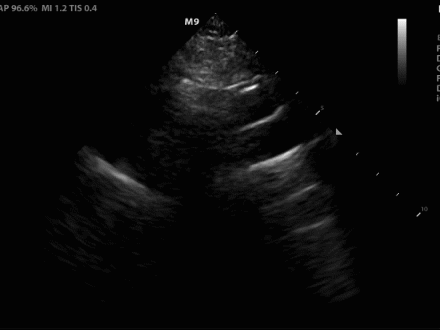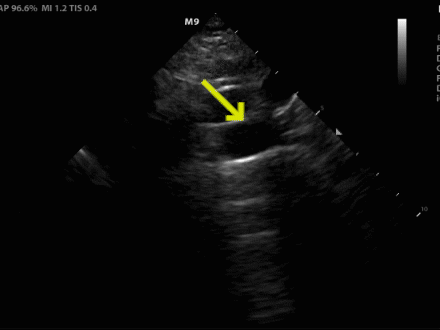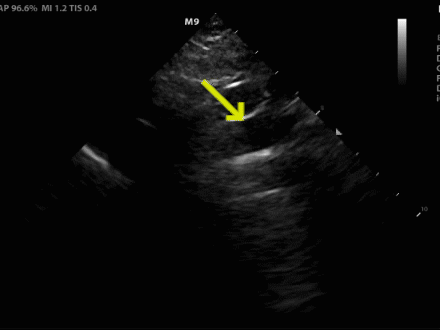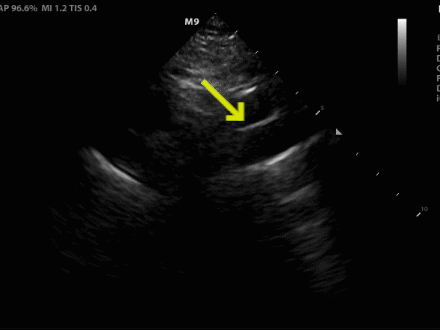
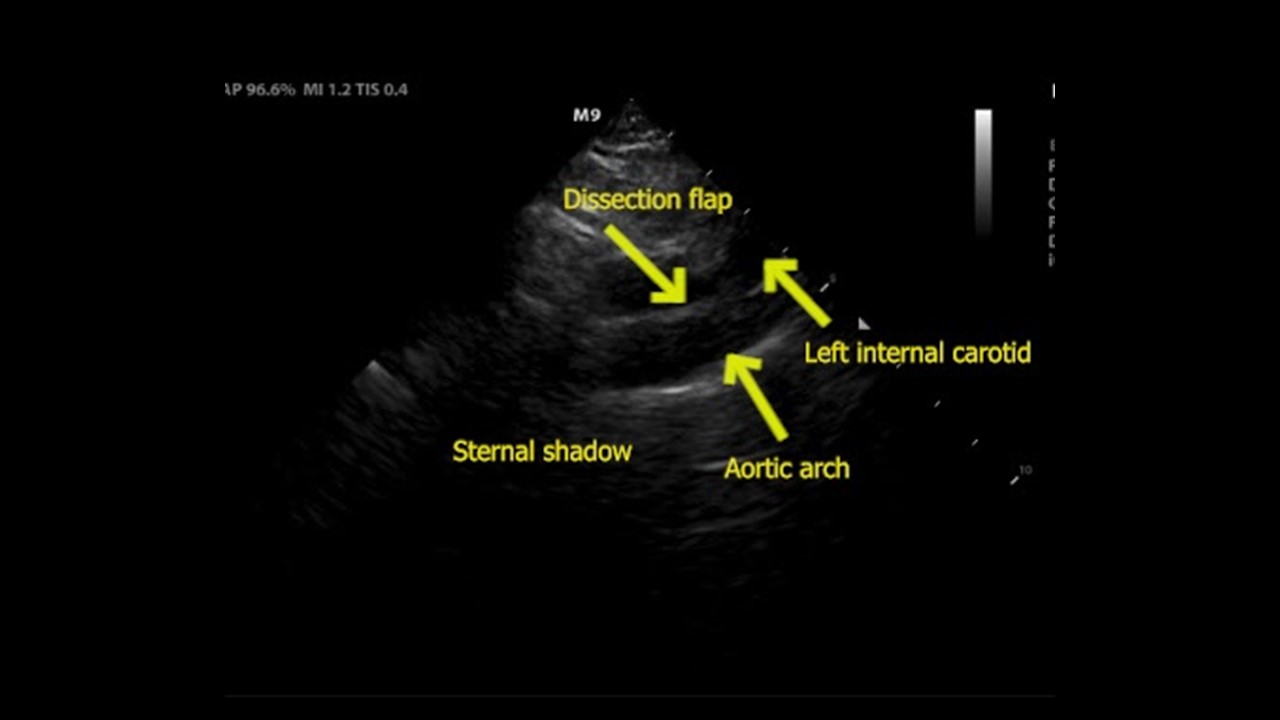
Figure 2. (A) Suprasternal notch view revealed a dissection flap (yellow arrow), confirming the suspected diagnosis.
Figure 2. (B) View with anatomical labels
Discussion
Acute aortic syndromes are a spectrum of disorders which can present in a myriad of ways, making them difficult to diagnose. Aortic dissection is one of these conditions which represents a time-sensitive diagnosis, and delaying treatment can lead to increased mortality.1 POCUS can be useful in expediting the diagnosis, treatment, and disposition in patients with acute aortic syndrome. Point-of-care echocardiography and ultrasound of the abdominal aorta are widely practiced by emergency physicians (EPs) and can reveal important aortic pathology. The parasternal long view evaluates the root of the aorta, and a diameter greater than 4 cm is suggestive of root dilation, which increases risk of aortic complications such as dissection, rupture, and valvular regurgitation.2
The suprasternal notch view (SSNV) allows visualization of the aortic arch, but is not performed often during routine transthoracic echocardiography.3 This view can be obtained by placing the phased array transducer along the suprasternal notch with the leading edge oriented to the patient’s right hip. Turning the patients head to the left or placing a rolled towel under the shoulders to extend the neck can optimize the view. One study evaluating EPs performing this view showed it was successfully obtained 97% of the time and rated as “easy” to obtain in 64.5% of cases and “difficult” in 7.6% of cases.4
Case Conclusion
Due to the POCUS findings, the patient was started on an esmolol drip for blood pressure control and the finding was confirmed with CT angiography. As the hospital did not have a cardiothoracic surgery service, the transfer process was initiated. While waiting for transfer, a nicardipine drip was started once the heart rate was below 100 bpm. The patient was then transferred to a nearby hospital where he had a successful surgery and recovery.
References
- Ohle R, Anjum O, Bleeker H, McIsaac S. What Is the Specificity of the Aortic Dissection Detection Risk Score in a Low-prevalence Population?. Acad Emerg Med. 2019;26(6):632-638. doi:10.1111/acem.13634
- Labovitz AJ, Noble VE, Bierig M, et al. Focused cardiac ultrasound in the emergent setting: a consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010;23(12):1225-1230. doi:10.1016/j.echo.2010.10.005
- Hussein A, Hilal D, Hamoui O, et al. Value of aortic arch analysis during routine transthoracic echocardiography in adults. Eur J Echocardiogr. 2009;10(5):625-629. doi:10.1093/ejechocard/jep014
- Kinnaman KA, Kimberly HH, Pivetta E, et al. Evaluation of the Aortic Arch from the Suprasternal Notch View Using Focused Cardiac Ultrasound. J Emerg Med. 2016;50(4):643-50.e1. doi:10.1016/j.jemermed.2015.12.002
Ultrasound Guided Procedures
Ultrasound Guided Popliteal Sciatic Nerve Block for a Complex Laceration on the Anterior Shin: A Case Report
Evan Yates, DO MBA; Avinash Viswanath, MD; Laura Riley, MD; Joseph Rodriguez, DO; and Getaw Hassen, MD PhD
Case
We present the case of a 45-year-old male who presented to the emergency department (ED) after falling into a manhole and sustaining a complex laceration to his right anterior lower leg. The patient had minimal pain relief after being given intravenous opioid medication. ED Providers then performed an ultrasound guided distal sciatic nerve block which brought the patient’s subjective pain rating at rest from 9/10 to 0/10. After the block was completed, the patient tolerated thorough irrigation and exploration of his wound without the need for further analgesia. The laceration was closed after requiring 2 cc of additional lidocaine to be injected to the medial aspect of his wound which was expected due to nerve distribution over the anterior lower leg. In patients with complex lower leg wounds, sciatic nerve blocks can be appropriate alternatives to local infiltration of analgesia providing greater pain relief with fewer intradermal injections and less overall opioid administration and reliance.
Introduction
Ultrasound guided nerve blocks are quickly becoming the mainstay treatment for numerous pathologies including biliary or renal colic, traumatic fractures or dislocations, or analgesia for wound management. This surge of ubiquity in ERs around the country, particularly in academic hospitals, is heightened by the opioid epidemic and by concerns about tipping the scales between appropriate and excessive opioid pain management possibly leading to dependence. By incorporating nerve blocks as adjunct or replacement therapy of traditional medical management, we can hope to see increases in the rates of overall pain control while simultaneously decreasing opioid prevalence.
The case described above illustrates the use of ultrasound guided nerve blocks for acute wound management as an appropriate alternative or adjunct analgesic therapy. The patient presented with a complex, deep wound to the anterior right shin after falling down a manhole (Figure 1).
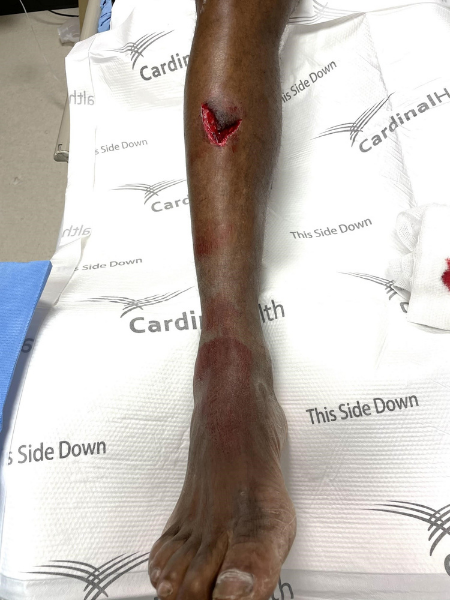
Figure 1. Wound on initial presentation
He was initially given a dose of IV morphine upon arrival given his severe pain and hesitancy to allow providers to even remove the dressing applied by EMS in the field. After obtaining consent, we performed an ultrasound guided popliteal sciatic nerve block (PSNB) to the injured extremity. Approximately 15 minutes after the block was completed we were able to thoroughly explore and irrigate the wound with no additional analgesia required.
Once the wound was explored to its full extent and sufficiently irrigated, wound closure was then addressed by an ED provider. At the point prior to closure, the patient continued to report a pain level of 0/10. An anchoring vertical mattress suture was first placed at the inferior apex of the wound without complication or elicitation of pain. Upon continuation of closure, the patient reported pain to the medial aspect of the laceration, but had no pain with closure of the lateral edge of the wound. Due to the pain, an additional 2 cc of 2% Lidocaine with epinephrine were injected locally and the wound was then successfully closed.
The PSNB adequately provided analgesia for this patient for the portion of his wound that was within the boundary of the superficial peroneal nerve. As we can see in Figure 2, it is likely that the medial aspect of his wound extended beyond this distribution, thus requiring a small amount of local infiltration.
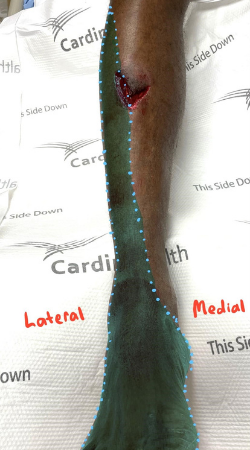
Figure 2. Approximate distribution of superficial peroneal nerve
Although the patient required this additional analgesia for wound closure, he continued to report 0/10 pain to his lower extremity, and was only given 1 dose of opioid medication prior to arrival. After successful wound closure, the patient was observed for 1 hour in the ED, seen ambulating with normal gait, and was discharged home without the need for additional pain medication.
Technique
The PSNB is typically performed from one of two positions, supine from a medial or lateral approach, or prone with an out of plane approach. Given the location of his wound, the decision was made for ease and comfort of the patient to perform the block using a supine lateral approach. Once consent was obtained from the patient, he was placed supine on his stretcher with his right leg propped up using sheets at the proximal thigh and middle calf. Using sterile technique, the right distal lateral thigh and knee was prepped and draped, and sterile ultrasound probe cover applied.
Under the supervision of an ultrasound fellowship-trained ED attending, and using a linear probe, we first obtained views from the popliteal fossa pointing the probe superiorly fanning caudally towards the superior aspect of the patella. First, the popliteal artery was identified with the popliteal vein lying just superiorly (Figure 3). The sciatic nerve was then identified superior to the pop. vein and followed to its division to the medial tibial nerve, and lateral common peroneal nerve (Figure 4).
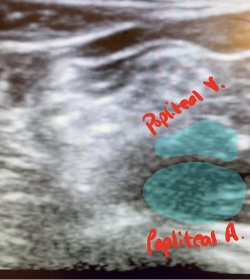
Figure 3. Identification of the popliteal artery and vein
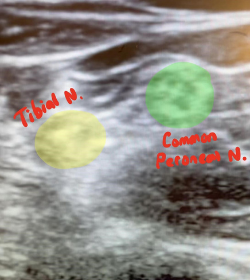
Figure 4. Identification of (medial) tibial nerve and (lateral) common peroneal nerve
Once these landmarks were identified, a 22 g spinal needle with extension tubing and a 20 cc syringe filled with 15 cc 2% lidocaine was introduced at the level of the proximal division of the sciatic nerve from the lateral aspect of the leg. Using ultrasound guidance, the needle tip was immediately identified and advanced to the level of sciatic nerve division. Using negative aspiration followed by incremental injections, the surrounding muscle and fascia were hydrodissected, and the remaining 12cc of Lidocaine was injected first between the tibial and common peroneal nerve in Vloka’s sheath (Figure 5), then circumferentially, with care being made to not pierce the nerve sheath. (Figure 6) The needle was then removed and the injection site covered with sterile gauze.
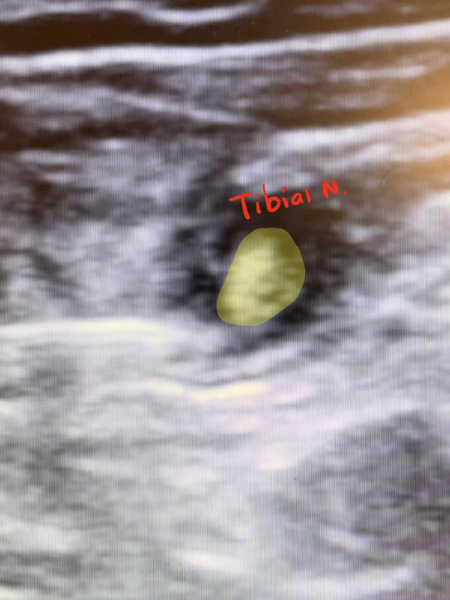
Figure 5. Hydrodissection of tibial nerve
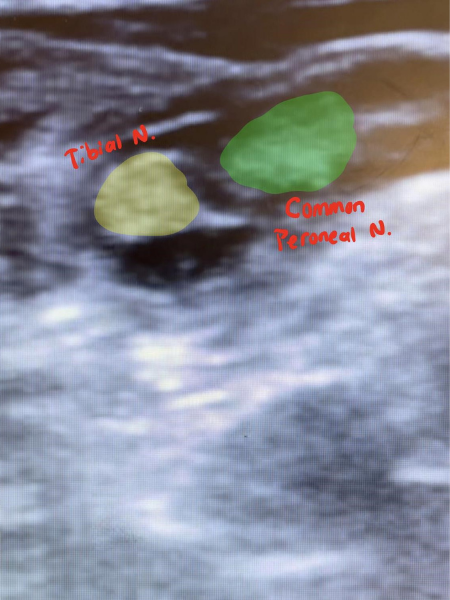
Figure 6. Completion of procedure, tibial and common peroneal nerves bathed in Lidocaine
Almost immediately, the patient reported pain relief.
Many studies have evaluated the efficacy of this block in terms of anesthetic placement and there seems to be general literature consensus that anesthesia has quicker onset and lasts longer when injected into Vloka’s sheath as opposed to the paraneural spaces. Vloka’s sheath is the area of connective tissue at the point between the common peroneal and tibial nerves seen just distal to their bifurcation from the sciatic nerve.
References
- Perlas A, Wong P, Abdallah F, et al. Ultrasound-guided popliteal nerve block through a common paraneural sheath versus conventional injection: a prospective, randomized, double-blind study. Reg Anesth Pain Med 2013;38:218–225.
doi:10.1097/AAP.0b013e31828db12f - Ip V and Tsui B: Injection through the paraneural sheath rather than circumferential spread facilitates safe, effective sciatic nerve block. Reg Anesth Pain Med 2013;38:37
doi:10.1097/AAP.0b013e318298671b - Lopez AM, Sala-Blanch X, Castillo R, et al. Ultrasound-guided injection inside the common sheath of the sciatic nerve at division level has a higher success rate than an injection outside the sheath. Rev Esp Anestesiol Reanim 2014;61:304–310.
doi:10.1016/j.redar.2013.11.018 - Choquet O, Noble GB, Abbal B, et al. Subparaneural versus circumferential extraneural injection at the bifurcation level in ultrasound-guided popliteal sciatic nerve blocks: a prospective, randomized, double-blind study. Reg Anesth Pain Med 2014;39:306–311.
doi:10.1097/AAP.0000000000000095 - Buys MJ, Arndt CD, Vagh F, et al. Ultrasound-guided sciatic nerve block in the popliteal fossa using a lateral approach: onset time comparing separate tibial and common peroneal nerve injections versus injecting proximal to the bifurcation. Anesth Analg 2010;110:635–637.
doi:10.1213/ANE.0b013e3181c88f27 - Vloka JD, Hadzić A, Lesser JB, et al. A common epineural sheath for the nerves in the popliteal fossa and its possible implications for sciatic nerve block. Anesth Analg 1997;84:387–390.
doi:10.1097/00000539-199702000-00028
Ultrasound Guided Brachial Plexus Nerve Block via The Retroclavicular Approach to the Infraclavicular Region (RAPTIR)
Chia-Yuan Michael Lee, DO; Jiodany Perez, MD; and Robert Farrow, DO
Case
A 49-year-old female presented to the emergency department complaining of pain and deformity of her left wrist after falling on outstretched hands while at the gym. Physical examination revealed a grossly deformed and tender left wrist (Figure 1) with a 2+ radial pulse, brisk capillary refill, and intact sensation. Radiographs of the left wrist demonstrated an acute, comminuted intra-articular fracture of the distal radius with severe loss of radial height, severe dorsal angulation, and 2 cm dorsal displacement (Figure 2).
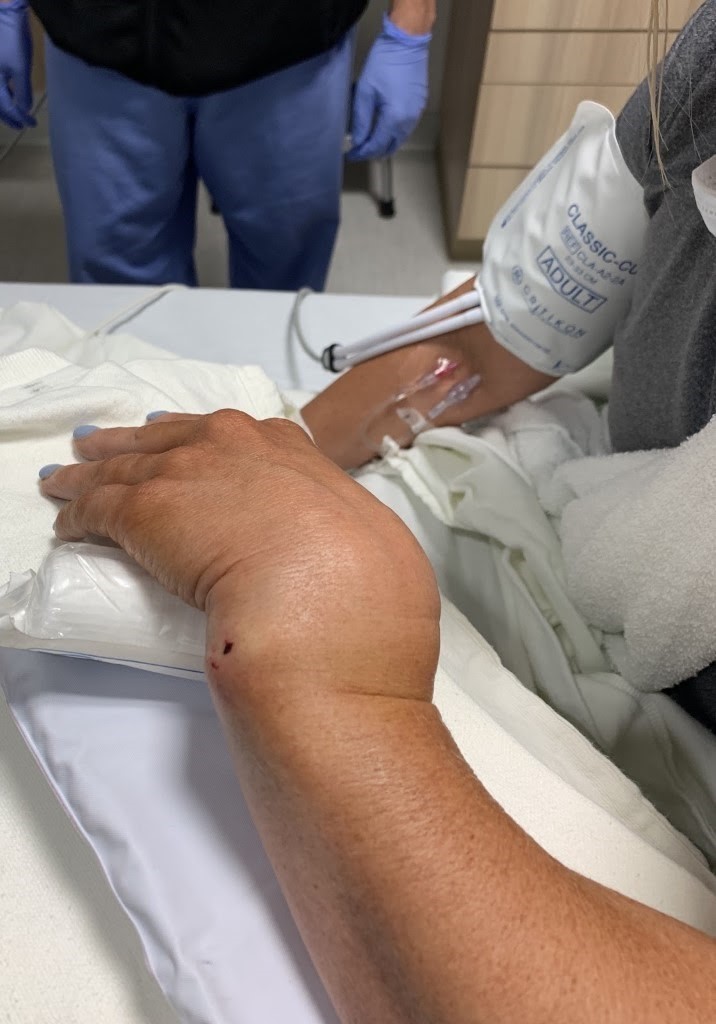
Figure 1. Gross Deformity of Patient’s Left Wrist
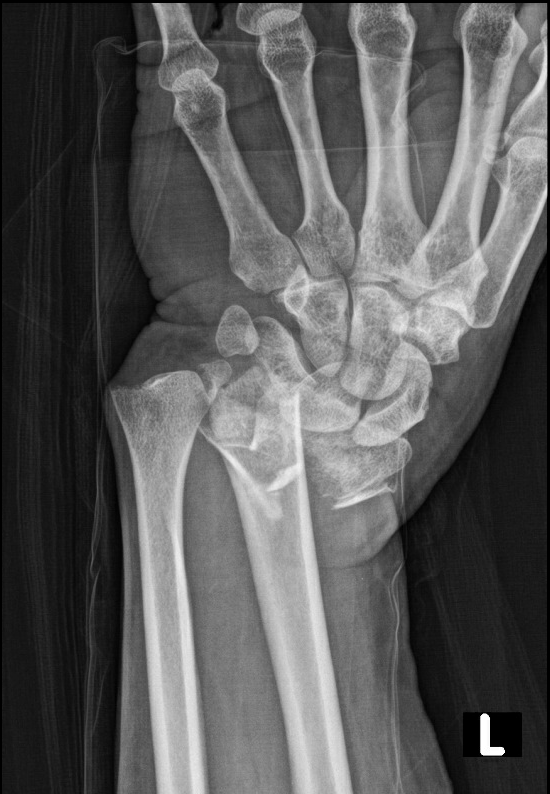
Figure 2. Pre-Reduction Radiograph of the Left Wrist
A brachial plexus nerve block using the retroclavicular approach to the infraclavicular region (RAPTIR) was performed to anesthetize the patient’s affected extremity which allowed for successful and painless closed fracture reduction with subsequent splint placement (Figure 3).
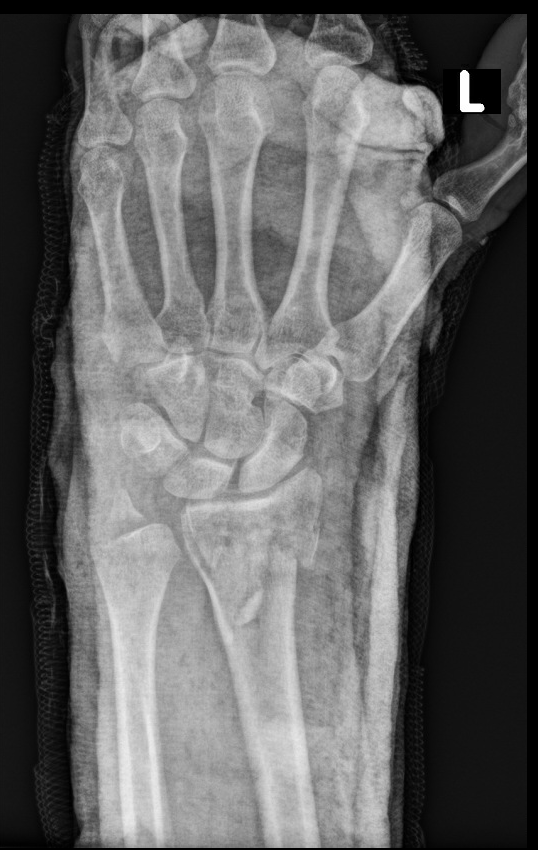
Figure 3. Post-Reduction Radiograph of the Left Wrist
The patient was discharged home in stable condition to follow-up with an orthopedic surgeon.
Introduction
Distal radius fractures are a common injury treated in emergency departments (ED) and are frequently caused by falling on an outstretched hand. Frequently, these injuries require reduction prior to splint application. Since reductions can be extremely painful, some form of analgesia is necessary. Certain medications may not be appropriate due to patient allergies, underlying comorbidities, or a desire to avoid narcotics. Although procedural sedation is an effective option, it typically requires assembly of multiple staff members, necessitates a prolonged post-procedural observation period, and carries the risk of hypotension and respiratory depression.1–3
Given these drawbacks, it is useful to have other options especially when significant reduction is anticipated. Nerve blocks are one such safe and effective alternative that typically do not require hemodynamic or airway monitoring or an extended observation period. Although nerve blocks in many cases can be performed “blind” using anatomical landmarks, the use of ultrasound (US) allows the operator to visualize structures, which facilitates performance of the block and minimizes the risk of potential complications such as hematoma formation or pneumothorax.1 Furthermore, in comparison to the blind approach, US-guided nerve blocks have a higher success rate, require less anesthetic, result in less damage to vessels, and achieve pain control more quickly.4
For management of distal radius fractures, one can block the brachial plexus, the network of nerves responsible for much of the innervation of the upper extremity. Brachial plexus nerve blocks have been noted to provide effective analgesia for reductions and even surgeries while decreasing length of ED stay in comparison to procedural sedation.1 Although there are multiple approaches to blocking the brachial plexus, the retroclavicular approach to the infraclavicular region (RAPTIR) is a relatively new technique that has shown promise in achieving analgesia for upper extremity procedures such as reductions and incision and drainage of abscesses.3,5 Below, we describe how to perform this procedure under ultrasound guidance.
Positioning and Scanning Technique 3,5,6
Begin the procedure by first positioning the patient either in a supine position or sitting with the head of the bed slightly elevated with arms adducted and the head turned/rotated to the contralateral side (Figure 4). A towel or blanket can be placed behind the ipsilateral shoulder for better exposure of the desired region. The US machine should be positioned in view directly in front of the operator on the ipsilateral side of the patient.
The RAPTIR nerve block requires the use of a high frequency linear array transducer probe (13-6 MHz). The US probe should be held with the operator’s non-dominant hand and a survey scan should be performed by sweeping medial to lateral from the middle 1/3 of the infraclavicular region with the probe marker pointing cephalad (Figure 4). The operator should sweep laterally while maintaining the same alignment until the axillary artery and vein emerge from behind the clavicle and an acoustic window is achieved between the clavicle and axillary artery. The survey scan is key for determining the ideal block location and also for identifying the major landmarks for the block: clavicle, pectoralis major, the axillary artery, nerve bundle, and the pleural line and ribs (Figure 5). It is critical to maintain all these structures in view while performing the procedure to minimize the risk of complications such as pneumothorax, inadvertent vasculature puncture, and direct trauma to the nerves.
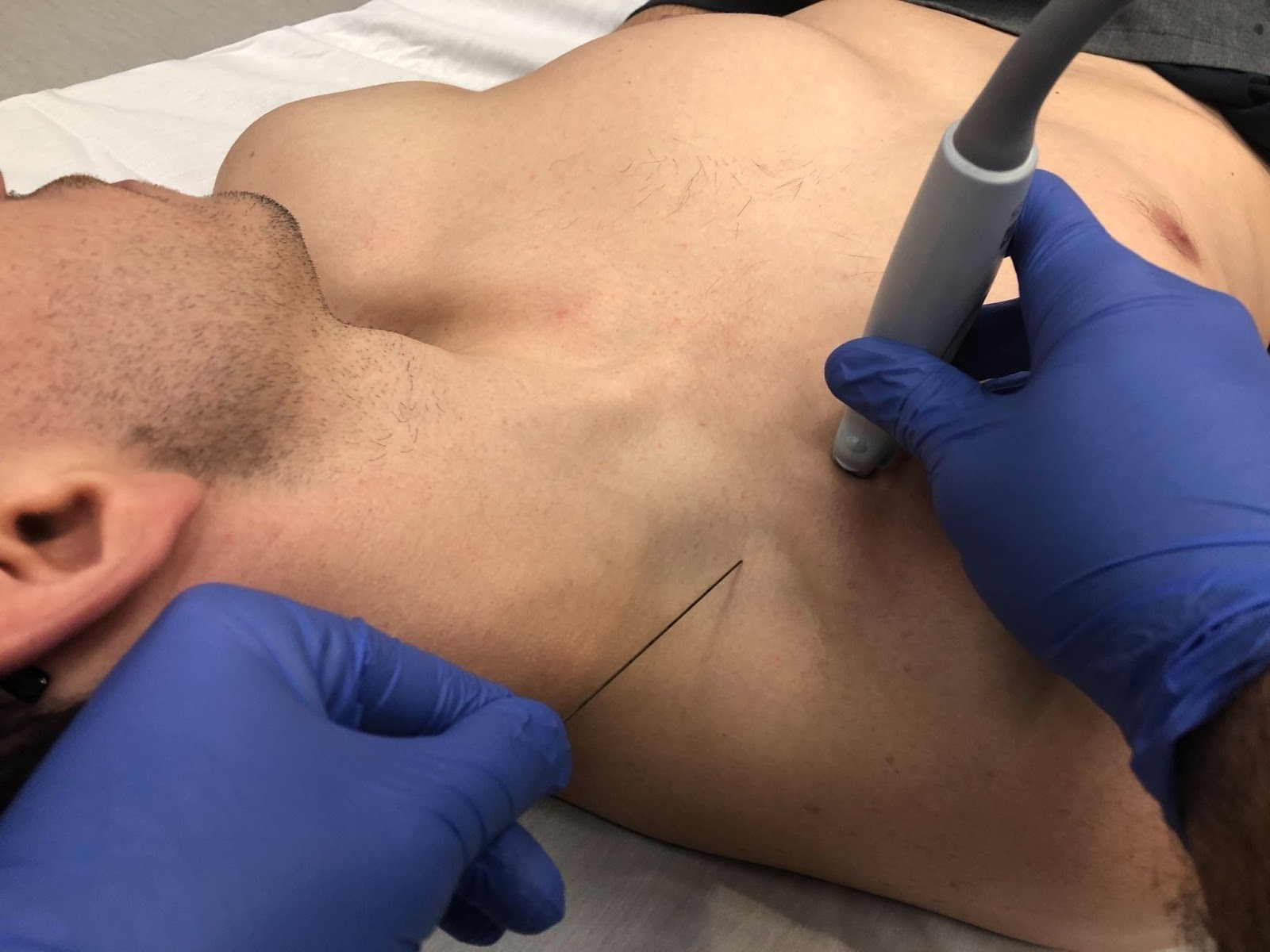
Figure 4. Positioning and scanning technique utilized for the RAPTIR nerve block.
The patient is lying in the supine position with arms adducted and head rotated to the contralateral side. The probe is positioned with probe marker facing cephalad. In this image, the area of needle insertion and the angle of insertion can be visualized.
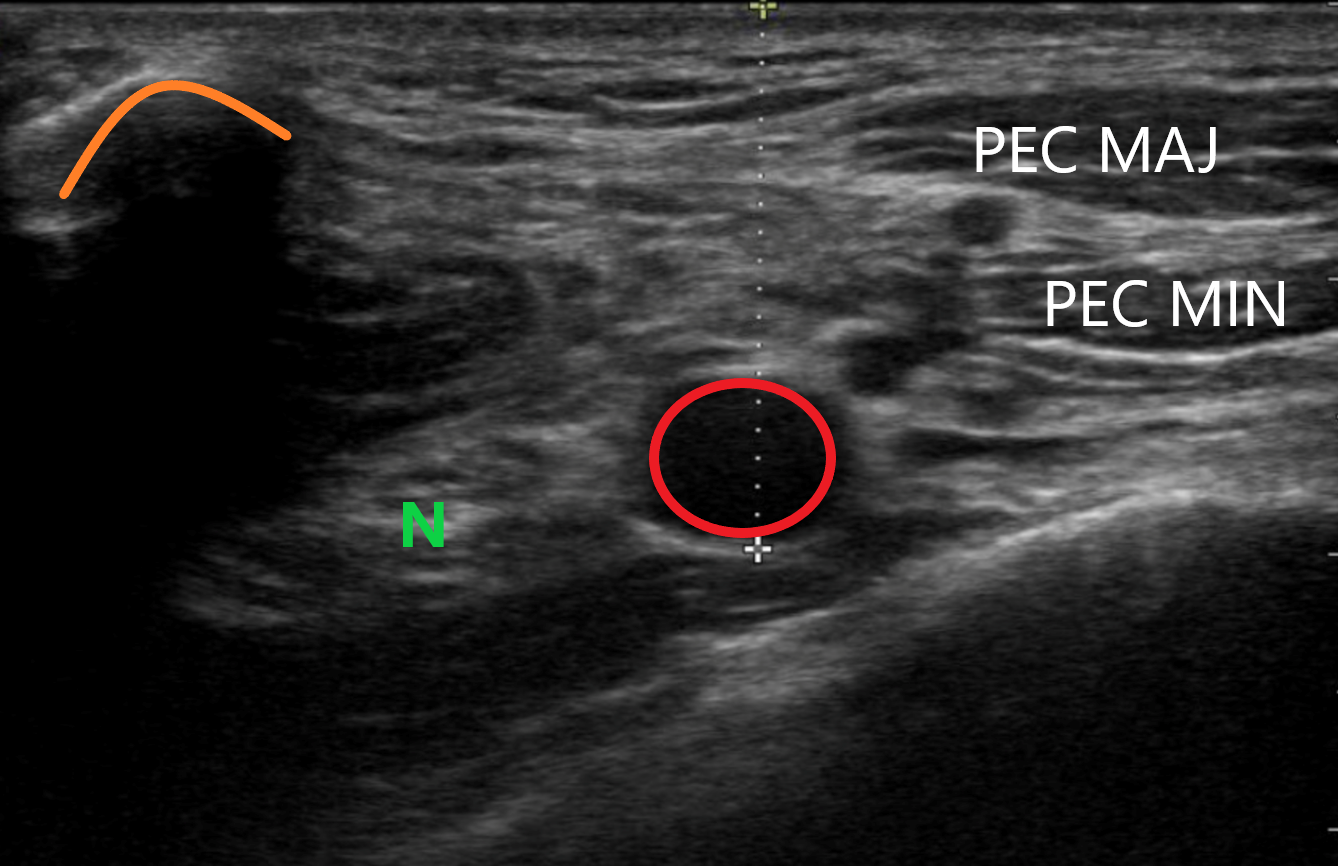
Figure 5. Ultrasound image of the desired region with the associated structures
The dotted line represents the measurement from the top of the screen to just beneath the axillary artery (red circle) which is flanked by the nerve bundle (N). Also highlighted are the clavicle (orange curve), rib (lime green line), pleural line (dotted blue line), pectoralis major (PEC MAJ) and pectoralis minor (PEC MIN).
Procedure 3,5,6
Once the key structures have been identified on the survey scan and the ideal block location has been selected, the operator should freeze the US image and measure down directly from the top of the US screen to just below the bottom of the axillary artery (Figure 5). This measurement represents how far down from the clavicle the needle needs to be inserted to maintain a flat and parallel approach to the US probe. Once the depth has been measured and skin appropriately cleaned for an aseptic procedure, the operator will then insert the needle in-line/in-plane to the US probe based on the measurement obtained in the previous step.
Advance the needle under the clavicle parallel to the probe and chest wall until the needle tip is visualized. Because the needle tip is not initially visualized until it passes under the clavicle, it is critical that the operator maintains a parallel position of the needle in respect to the chest wall and transducer without changing the angle or direction of advancement until the needle tip is fully visualized.
Once the needle tip is identified along with the key structures, use gentle hydro-dissection with sterile normal saline (NS) to slowly advance the needle to just below the axillary artery. Aspirate to confirm that arterial structures were not inadvertently punctured and then continue the procedure by gently injecting sterile NS to the region just deep to the axillary artery.
After the anechoic/hypoechoic fluid lifts the axillary artery and spreads to either side, the appropriate position has been established and administration of the weight-based dose of LA may be injected (Figure 6). The RAPTIR is a high-volume block as it depends on a fascial plane to distribute the anesthetic over the nerve cords surrounding the axillary artery. Typically, the operator will need to supplement the weight-based volume of LA with sterile NS to achieve total volumes of approximately 40-60 cc.
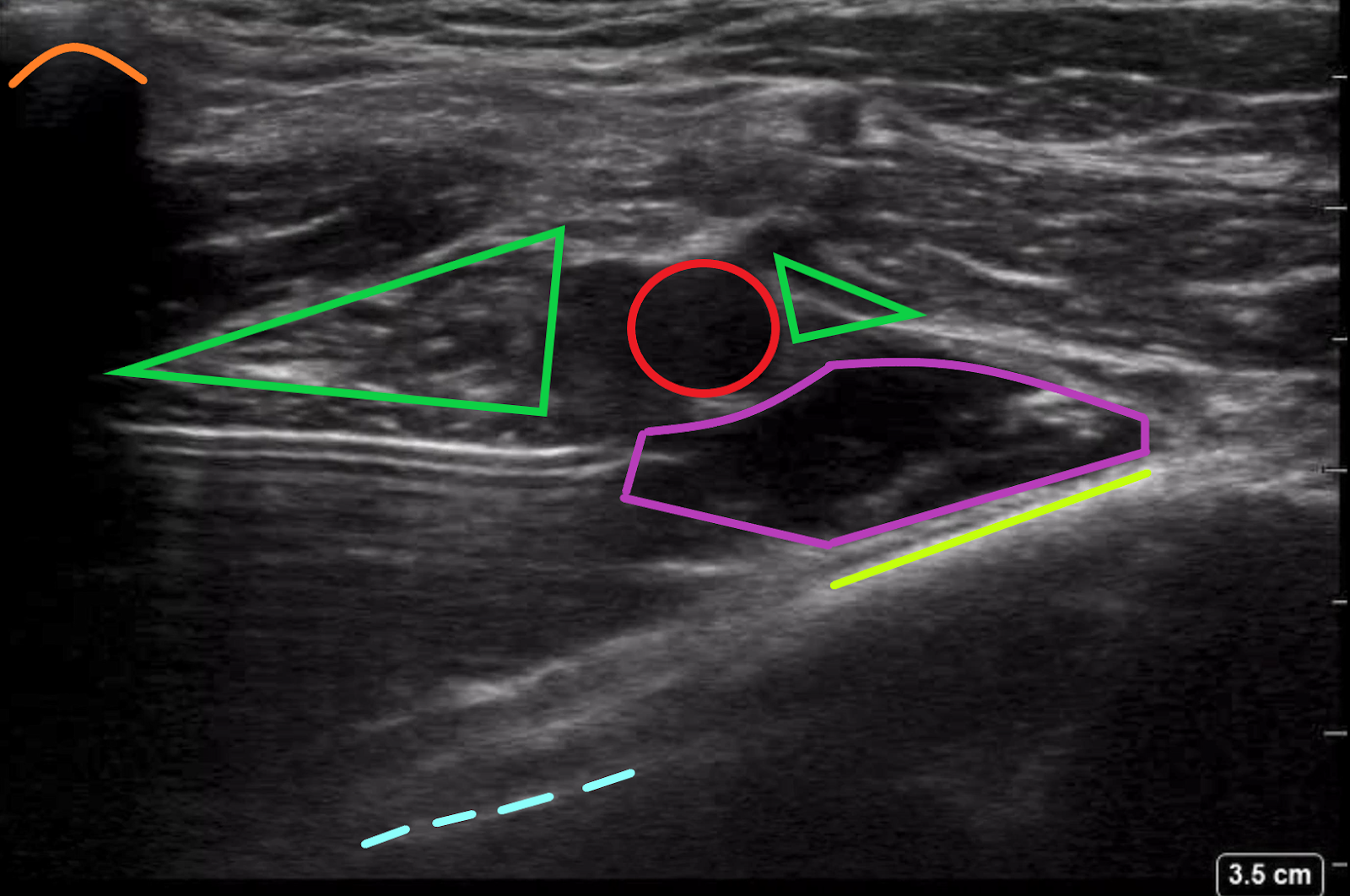
Figure 6. Ultrasound image of needle insertion and local anesthetic administration
The local anesthetic, outlined in purple, is being administered just underneath the axillary artery (red circle) which lifts the nerve bundle (green triangles). Also visible is the pleural line (dotted blue line), rib (lime green line), and the clavicle (orange curve).
References
- Bhoi S, Chandra A, Galwankar S. Ultrasound-guided nerve blocks in the emergency department. Journal of Emergencies, Trauma and Shock. 2010;3(1):82-88. doi:10.4103/0974-2700.58655
- Nejat A, Teymourian H, Behrooz L, et al. Pain management via ultrasound-guided nerve block in emergency department; A case series study. Archives of Academic Emergency Medicine. 2019;7(1):12. doi:10.22037/emergency.v5i1.12642
- Luftig J, Mantuani D, Herring AA, et al. Ultrasound-guided retroclavicular approach infraclavicular brachial plexus block for upper extremity emergency procedures. American Journal of Emergency Medicine. 2017;35(5):773-777. doi:10.1016/j.ajem.2017.01.028
- Canders C, Morales D, Sha S. Ultrasound-guided Nerve Blocks in the Emergency Department. Published March 1, 2018. Accessed March 27, 2021. https://www.reliasmedia.com/articles/142305-ultrasound-guided-nerve-blocks-in-the-emergency-department
- Gelber J, Luftig J, Mantuani D. Ultrasound-Guided Retroclavicular Approach to the Infraclavicular Region (RAPTIR) Anesthesia for Challenging Upper Extremity Reductions. Journal of Emergency Medicine. Published online 2021. doi:10.1016/j.jemermed.2021.01.007
- Pester JM, Varacallo M. Brachial Plexus Block Techniques. StatPearls Publishing; 2019. Accessed March 27, 2021. http://www.ncbi.nlm.nih.gov/pubmed/29262036
Ultrasound Saves
Musculoskeletal Ultrasound Guided Needle Aspiration of a Septic Subdeltoid Bursitis
Jiodany Perez, MD and Michael Rosselli, MD MPH
The Case
A 79-year-old male with a past medical history significant for diabetes mellitus, hypertension, and coronary artery disease s/p ST-elevation myocardial infarction with stent placement three days ago presented to the emergency department complaining of fever. The patient stated that he sought medical care in the emergency department today because he was concerned that he was infected with the COVID-19 virus. Given his possible exposure during a recent hospital admission just three days prior for stent placement, he believed himself to be at increased risk. The patient otherwise denied recent trauma, rash, travel or sick contacts at home. Review of systems was positive for mild shortness of breath, chills, myalgias, and arthralgias for the past two days.
His physical exam was remarkable for vital signs of; blood pressure 122/62 mmHg, pulse of 103 beats per minute, oral temperature of 38.4 degrees Celsius, respiratory rate of 21 breaths per minute, and an O2 saturation of 93% on room air. The patient was not in acute distress on initial evaluation. He was found to be tachypneic with lung sounds clear to auscultation bilaterally. Cardiac exam demonstrated a tachycardic regular rhythm, pulses were 2+ in the bilateral radial, dorsalis pedis, and posterior tibial arteries. Musculoskeletal examination was remarkable for slight tenderness to palpation of the anterior and lateral aspect of his right shoulder. He had decreased range of motion of his right shoulder in abduction, flexion, and extension with associated pain.
Point of care ultrasound (POCUS) using a 15-6 MHz wide-footprint linear transducer was selected for further evaluation of the right posterior shoulder girdle given the patient’s initial complaints and PE findings. Noted during the POCUS evaluation was a distended, hypoechoic subdeltoid bursa, along with a glenohumeral joint effusion. Communication between these two fluid collections was seen, suggesting that a significant rotator cuff tear was present.
Ultrasound-guided needle aspiration of the right subdeltoid bursa was subsequently performed (Figure 3), which yielded 20 mL of yellow purulent fluid. Fluid analysis demonstrated 199,294 nucleated cells/microliter. The patient was treated in the emergency department with 1,750 mg of vancomycin and 2 g of cefepime intravenously and the patient was admitted with the diagnosis of septic bursitis. Cultures of this aspirate grew methicillin resistant staphylococcus aureus, which were also present in the patient’s blood cultures on day 1 of hospitalization.
Background
Mortality rates for septic arthritis have been documented to be between 3-25%.1-4 The incidence of septic arthritis varies in the literature; however, 4-60 cases per 100,000 population per year is suggested.1-5 he incidence of septic arthritis has been climbing, with a reported rise of 43% between 1998-2013 (5.5/100,000 to 7.3/100,000). The largest increase in incidence was noted to be in patients > 75 years of age.4
The primary symptoms of this disease process include mono-articular joint pain, erythema, warmth, and limited mobility. However, these symptoms have a broad differential diagnosis; including trauma, crystalloid arthropathy, rheumatoid arthritis, avascular necrosis, Lyme disease, osteomyelitis, malignancy, transient synovitis, lupus, and others.6 The associated constitutional symptoms of fever, chills, and rigors may help in making the diagnosis if present. History and clinical exam findings alone have been found to be poorly sensitive for the diagnosis of acute septic arthritis.7
Further complicating the early recognition of this infectious process is the COVID-19 pandemic. Multiple case reports have demonstrated how cognitive bias has led to the delayed diagnosis and treatment of time sensitive disease processes that present with similar symptoms as a COVID-19 viral illness.8-9 The use of MSK POCUS has been shown to be an effective tool in the diagnosis of both septic arthritis and septic bursitis. MSK POCUS not only allows one to directly image the joint and bursa affected, but it improves the success rate of needle-guided arthrocentesis.10 The latter is critical in establishing the definitive diagnosis, as identifying an offending organism via synovial fluid culture will distinguish septic arthritis from the other arthropathies listed above.11
Scanning technique and procedure
Start by collecting the supplies necessary for the procedure. Supplies:
- Portable Ultrasound Machine with 15-6 MHz wide-footprint linear transducer
- 18 gauge needle
- 20 cc syringe
- Lidocaine 1% without epinephrine
- Sterile specimen collecting cup
- Skin anti-septic solution
- Sterile gloves
- Bandage
Once your supplies have been collected, position the patient sitting upright with the arm adducted and internally rotated, as illustrated in Figure 1. Place the probe in a transverse position along the posterior shoulder girdle until the desired structures are identified; including, the posterior deltoid, the sub-deltoid bursa, the humeral head and the gleno-humeral joint (Figure 2). Using the tip-toe technique optimize your image so all desired structures are in view during the procedure. Once the structures have been identified insert your needle, in plane, at an approximately 45-60 degree angle just lateral to the edge of the probe (Figure 1). Visualize your needle inserting into the skin and maintain in-plane alignment with the probe. Locate your needle tip on the screen and advance the needle tip until it is seen within the hypoechoic bursa (Figure 3). Aspiration of the bursal fluid should be performed with an 18 gauge needle attached to a 20cc syringe.
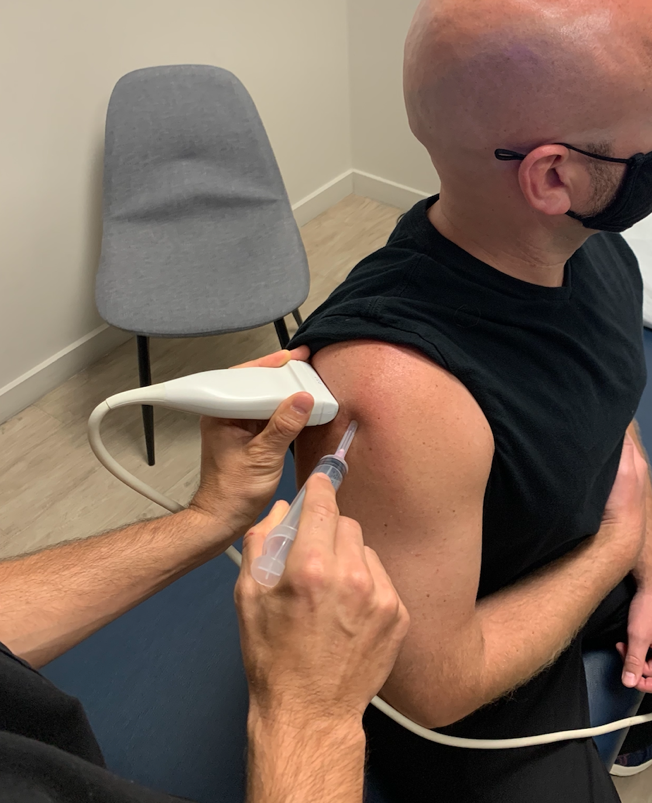
Figure 1. Patient positioned sitting up right with their upper extremity adducted and internally rotated. In this image, the transducer probe is positioned in the posterior shoulder girdle in the transverse position. The appropriate needle insertion site and angle of insertion are also being demonstrated.
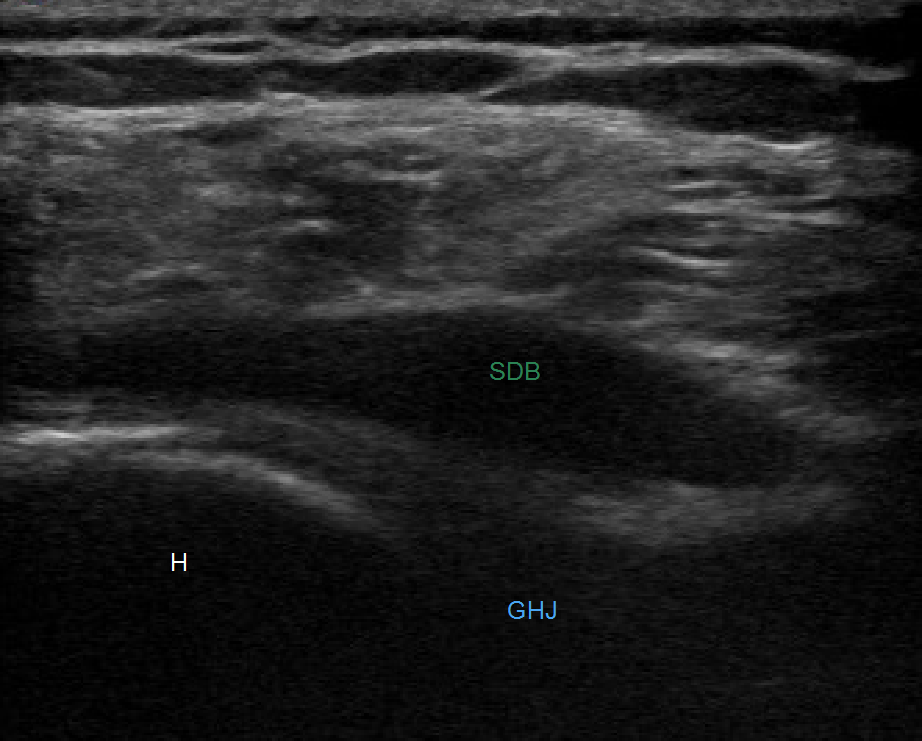
Figure 2. US evaluation of the right subdeltoid bursa revealing a distended hypoechoic subdeltoid bursa (Green SDB) and a glenohumeral joint effusion (Blue GHJ). The humeral head is also identified in this image (H).
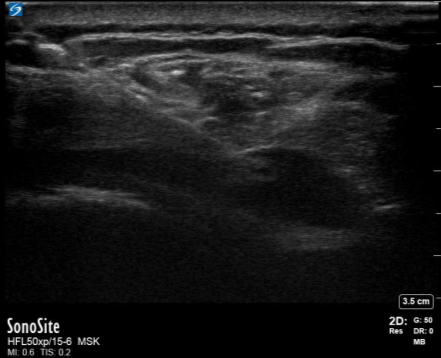
Figure 3. US guided needle aspiration of the right subdeltoid bursa.
References
- Goldenberg, D. L. (1989). Infectious arthritis complicating rheumatoid arthritis and other chronic rheumatic disorders. Arthritis & Rheumatism, 32(4), 496-502.
- Kaandorp CJ, Dinant HJ, van de Laar MA, et al. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann Rheum Dis. 1997;56(8):470–5.
- Cooper C, Cawley MI. Bacterial arthritis in an English health district: a 10-year review. Ann Rheum Dis. 1986;45(6):458–63.
- Rutherford AI, Subesinghe S, Bharucha T, et al. Galloway, A population study of the reported incidence of native joint septic arthritis in the United Kingdom between 1998 and 2013, Rheumatology, 55(12): 2176–2180.
- Morgan DS, Fisher D, Merianos A, et al. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect. 1996;117(3):423–8.
- Long B, Koyfman A, Gottlieb M. Evaluation and Management of Septic Arthritis and its Mimics in the Emergency Department. West J Emerg Med. 2019 Mar;20(2):331-341.
- Carpenter CR, Schuur JD, Everett WW, et al. Evidence-based diagnostics: adult septic arthritis. Acad Emerg Med. 2011;18(8):781–96.
- Harada T, Watari T, Miyagami T, et al. COVID Blindness: Delayed Diagnosis of Aseptic Meningitis in the COVID-19 Era. Eur J Case Rep Intern Med. 2020;7(11):001940.
- Harada Y, Shimizu T. Delayed Diagnosis of Pulmonary Thromboembolism Due to Overfocus on COVID-19. Eur J Case Rep Intern Med. 2020;7(11):002002.
- Papatheodorou A, Ellinas P, Takis F, et al. US of the shoulder: rotator cuff and non-rotator cuff disorders. Radiographics. 2006;26(1):e23.
- Horowitz DL, Katzap E, Horowitz S, et al. Approach to septic arthritis. Am Fam Physician. 2011;84(6):653-60.
Evaluation for Bladder Rupture
Taylor Bashara, MS3; Matthew Flannigan, DO; and Sara Urquhart, MS3
Michigan State University College of Human Medicine
Introduction
Bladder ruptures are a relatively uncommon injury following blunt abdominal trauma.1,2 Bladder ruptures are classified as intraperitoneal or extraperitoneal injuries.3,4 Extraperitoneal bladder ruptures account for the majority of bladder ruptures and are treated easily with a Foley catheter. Intraperitoneal ruptures are often a result of motor vehicle collisions resulting in pelvic fractures and require a surgical intervention. A suspected bladder rupture is commonly diagnosed with cystography using plain film, fluoroscopy, or computerized tomography (CT).4,5 An extended focused assessment with sonography (EFAST) has been recognized to be a useful screening exam that improves the early recognition of blunt traumatic injuries, including bladder rupture.6,7
Patient Description
A 59-year-old male was brought initially to an outside level III trauma center by ambulance after a motorcycle accident in which the patient hit an embankment and was thrown from his motorcycle, landing several feet away. On initial assessment, the patient was hypotensive and complained of back and rib pain. Vital signs were: HR 123, BP 62/41,RR 23, SpO2 97%. Physical exam revealed: GCS 15; lungs: clear without respiratory distress; abdomen: firm, nondistended, no suprapubic tenderness. He was given 3 L of normal saline for suspected intraperitoneal bleeding. The patient had whole body CT imaging, which identified lumbar fractures (L1-L3) and multiple pelvic fractures: right superior and inferior pubic rami with widening of the pubic symphysis, right sacral ala, and bilateral sacrococcygeal. His CT abdomen/pelvis demonstrated no obvious intraparenchymal injury, free fluid, or apparent bladder injury.
The patient was transferred to a level I trauma center. On arrival, the patient was hypotensive and complained of shortness of breath, back and pelvic pain, and a headache. Vital signs: HR 112, BP 73/45,RR 26, SpO2 96%. Physical exam revealed: GCS 15; airway intact, cervical collar in place; CV: regular rate and rhythm, 2+ DP, PT, and radial pulses bilaterally; thorax: right anterior chest wall tenderness; abdomen/pelvis: soft, nontender, pelvis stable, blood at urethral meatus, perineal ecchymosis; MSK: midline lumbar spine tenderness without step-offs; neurological: A/O x 4, moves all 4 extremities, sensation intact to light touch.
Intervention
An e-FAST exam was performed, demonstrating a new onset free fluid in the left upper quadrant (Figure 1).
Figure 1. EFAST – Left Upper Quadrant
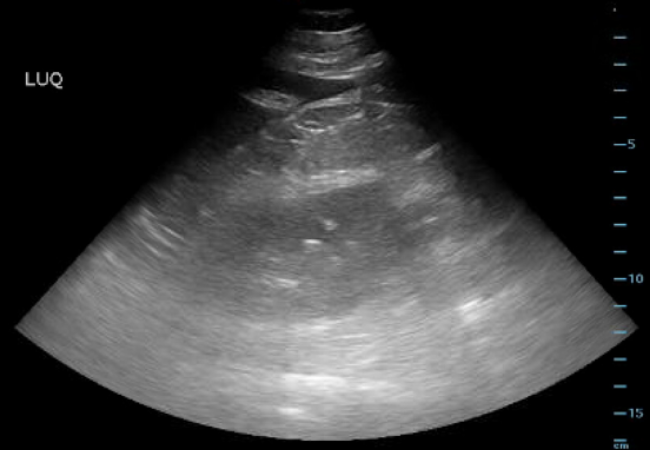
The LUQ demonstrated intra-abdominal free fluid.
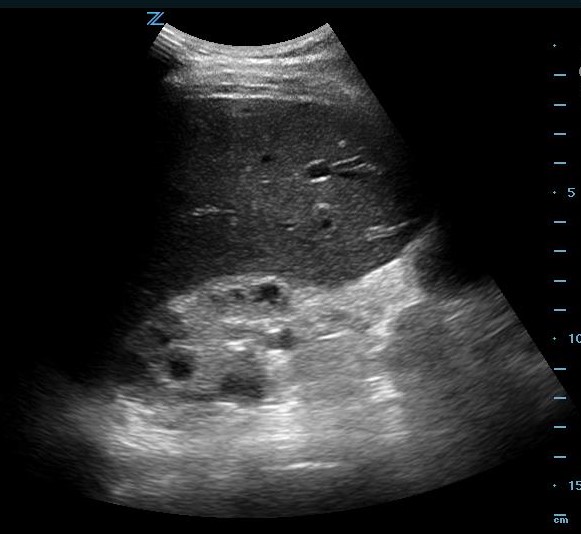
The pelvic view was not evaluated due to the presence of a pelvic binder. The patient was stabilized with an additional 2 L of lactated ringers and 2 units of packed RBCs. He was transported for an additional abdominal and pelvis CT due to his hemodynamic deterioration and POCUS detection of intraperitoneal fluid. The repeat CT scan demonstrated an intraperitoneal bladder rupture in addition to the previously identified pelvic and lumbar spine fractures.
Figure 2. Post-EFAST Computed Tomography (CT) Scan
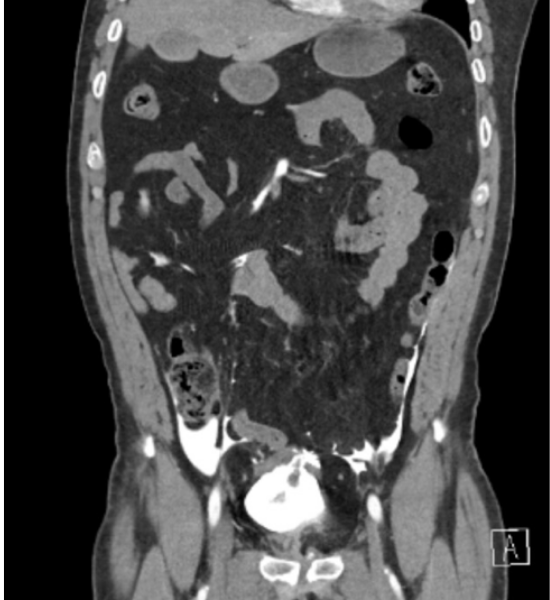
The post-EFAST CT revealed intraperitoneal bladder rupture.
The patient was taken for an exploratory laparotomy. He was found to have a 5 cm bladder dome laceration, a grade one splenic laceration, and a RLQ mesenteric hematoma. Post-operatively the patient was transferred to the intensive care unit and eventually discharged to an acute rehabilitation facility on post-op day 5.
Conclusion
Bladder ruptures are often not visible on initial CT, commonly due to incomplete bladder distention and/or inadequate filling of the bladder by contrast.1 In an optimal setting, both conventional and CT cystography can detect bladder ruptures with a sensitivity and specificity as high as 95% and 100%, respectively.8-10 Furthermore, e-FAST has been reported to have a sensitivity of 74% and a specificity of 98% for intra-abdominal free fluid.11 In patients who present after blunt trauma and are found to have pelvic fractures, it is important to consider the possibility of an intraperitoneal bladder rupture as a potential source for intraperitoneal free fluid. An e-FAST that demonstrates echogenic debris (blood) within the bladder and intraperitoneal fluid are potential clues for a genitourinary injury.
References
- Barnard, J., Overholt, T., Hajiran, A., et al. (2019). Traumatic Bladder Ruptures: A Ten-Year Review at a Level 1 Trauma Center. Advances in Urology, 2019, 2614586.
- Mahat, Y., Leong, J. Y., & Chung, P. H. (2019). A contemporary review of adult bladder trauma. Journal of Injury and Violence Research, 11(2), 101–106.
- Gross, J. S., Rotenberg, S., & Horrow, M. M. (2014). Resident and Fellow Education Feature Bladder Injury: Types, Mechanisms, and Diagnostic Imaging. RadioGraphics, 34(3), 802–803.
- Simon, L. V., Sajjad, H., Lopez, R. A., et al. (2020). Bladder Rupture. In StatPearls. StatPearls Publishing.
- Rose, J. S. (2004). Ultrasound in abdominal trauma. Emergency Medicine Clinics of North America, 22(3), 581–599.
- Patel, N. Y., & Riherd, J. M. (2011). Focused Assessment with Sonography for Trauma: Methods, Accuracy, and Indications. Surgical Clinics of North America, 91(1), 195–207.
- Richards, J. R., & McGahan, J. P. (2017). Focused Assessment with Sonography in Trauma (FAST) in 2017: What Radiologists Can Learn. Radiology, 283(1), 30–48.
- Chan, D. P. N., Abujudeh, H. H., Cushing, G. L., et al. (2006). CT cystography with multiplanar reformation for suspected bladder rupture: Experience in 234 cases. AJR. American Journal of Roentgenology, 187(5), 1296–1302.
- Ishak, C., & Kanth, N. (2011). Bladder trauma: Multidetector computed tomography cystography. Emergency Radiology, 18(4), 321–327.
- Quagliano, P. V., Delair, S. M., & Malhotra, A. K. (2006). Diagnosis of blunt bladder injury: A prospective comparative study of computed tomography cystography and conventional retrograde cystography. The Journal of Trauma, 61(2), 410–421.
- Netherton, S., Milenkovic, V., Taylor, M., et al. (2019). Diagnostic accuracy of eFAST in the trauma patient: A systematic review and meta-analysis. CJEM, 21(6), 727–738.