Fall 2020
Welcome from EUS-AAEM
The Emergency Ultrasound Section of the American Academy of Emergency Medicine (EUS-AAEM) is founded to foster the professional development of its members and to educate them regarding point-of-care ultrasound. This group will serve as a venue for collaboration among medical students, residents and practitioners who are interested in point-of-care ultrasound. The purpose of our group is to augment the knowledge and expertise of all emergency medicine specialists and to advocate for patient safety and quality care by endorsing bedside ultrasound. Membership is not limited to fellowship trained physicians. All emergency medicine practitioners passionate about ultrasound are welcome to join and participate.
We are proud to publish our e-newsletter with original contributions from many of our members. We encourage all members to submit for future editions. Topics include but are not limited to educational, community focus, interesting cases, resident and student section, and adventures abroad.
For more information visit us online at: www.aaem.org/EUS.
In this Issue:
-
President's Message
-
Ultrasound Building Blocks: Right Upper Quadrant Pain and Free Pericholecystic Fluid Are Not Always Cholecystitis
-
Ultrasound Building Blocks: POCUS, on the Rocks!
-
Resident Section: Retrobulbar Hematoma
-
Ultrasound Highlights: Massive PE and More
-
Ultrasound Highlights: The Aortic Flap Dance
-
Ultrasound Highlights: Always Grateful for a Water Bath
-
Fellow Section: An Unusual Case of Adult Intussusception
-
US Guided Procedures: Ultrasound Guided Nerve Block of the Posterior Tibial Nerve
-
US Guided Procedures: Traumatic Knee Joint Aspiration
-
Administrative Corner: System-Wide Clinical Ultrasound Committees
-
Ultrasound Saves: Cardiac Tamponade Secondary to Pacemaker Perforation of the Myocardium: A Case Report
-
Ultrasound Saves: Ultrasound-guided Pericardiocentesis
Welcome from EUS-AAEM!
The Emergency Ultrasound Section of the American Academy of Emergency Medicine (EUS-AAEM) is founded to foster the professional development of its members and to educate them regarding point-of-care ultrasound. This group will serve as a venue for collaboration among medical students, residents, and practitioners who are interested in point-of-care ultrasound. The purpose of our group is to augment the knowledge and expertise of all emergency medicine specialists and to advocate for patient safety and quality care by endorsing bedside ultrasound. Membership is not limited to fellowship-trained physicians. All emergency medicine practitioners passionate about ultrasound are welcome to join and participate.
We are proud to publish our e-newsletter with original contributions from many of our members. We encourage all members to submit for future additions. Topics include but are not limited to educational, community focus, interesting cases, resident and student section, and adventures abroad.
For more information, visit us online at: www.aaem.org/EUS.
President's Message
Hello EUS-AAEM Members,
The year 2020 has been challenging for all of emergency medicine. With the COVID-19 pandemic, ultrasound has been more important than ever. Studies have begun to demonstrate the increasing role of ultrasound in diagnosing COVID-19 and its complications. Moreover, as there are efforts to reduce transportation of patients and potential exposure of additional health care workers, point-of-care ultrasound is again at the forefront of providing care to these patients.
The EUS-AAM section is working to provide ultrasound education during the pandemic. As many local courses have been cancelled, we are planning to launch two separate yearlong virtual ultrasound courses for our members. These will include both a beginner course and an advanced course, with topics ranging from basic cardiac to advanced nerve blocks and bowel ultrasound. This will feature engaging and interactive talks given by national experts and include a live question and answer session at the end. We are also collaborating with ACEP, SAEM, and SCUF to create a curated resource for ultrasound education.
In the meantime, we hope that you will enjoy the articles included in this issue. We will be discussing a variety of topics including system-wide clinical ultrasound committees, massive pulmonary embolism, ultrasound-guided procedures, and more.
Finally, there are many ways to get involved with EUS-AAEM. Please reach out if you would like to learn more.
Sincerely,
Michael Gottlieb, MD RDMS FAAEM
EUS-AAEM President 2020-21
Ultrasound Building Blocks
Right Upper Quadrant Pain and Free Pericholecystic Fluid Are Not Always Cholecystitis
Samuel Barnett, MD and Monika Lusiak, MD
Case Description
A 65-year-old female with a past medical history of breast cancer in remission presents to the ED with one day of worsening right-sided abdominal pain, nausea, and NBNB emesis. She was seen by her PCP the previous day for similar complaints and was prescribed a PPI for suspected gastritis; her symptoms have since worsened. She denies fever, diarrhea, dysuria, urinary frequency, chest pain, or shortness of breath. On exam, the patient appears ill and in distress lying in bed. She is afebrile with regular sinus tachycardia, a non-distended abdomen, and moderate-severe tenderness worst in RUQ with rebound. Sclerae are anicteric.
Labs show no leukocytosis, elevated lactate at 5.1, normal lipase, and elevated transaminases with AST 113, ALT 235, Alk phos 240, TBili 0.5.
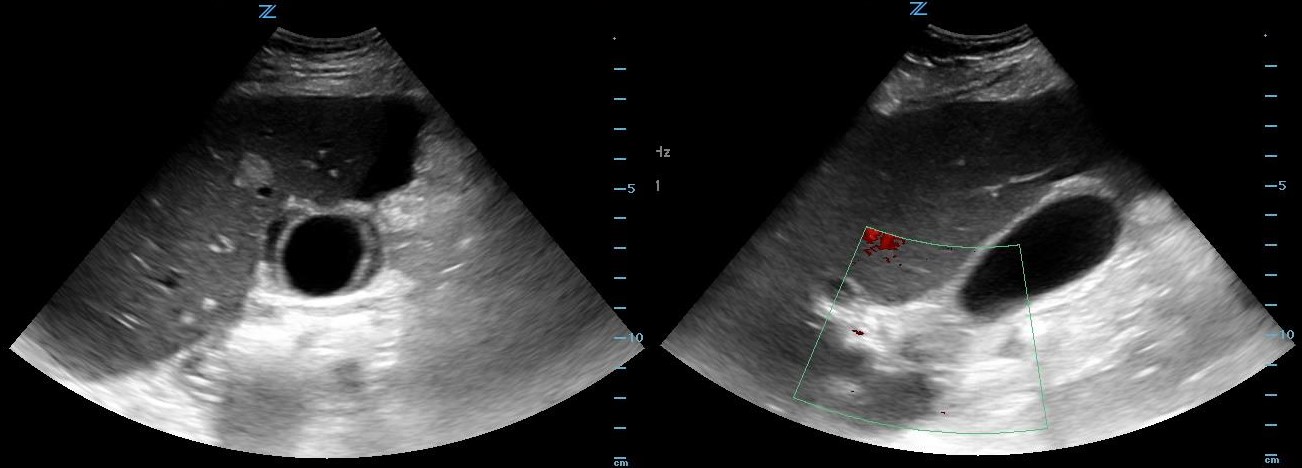
POCUS of RUQ shows free pericholecystic fluid, normal size GB without stones, no wall edema, and dilated CBD with positive sonographic Murphy’s sign. (Figure 1)
Figure 1. Transverse and long axis views of gallbladder demonstrating free pericholecystic fluid.
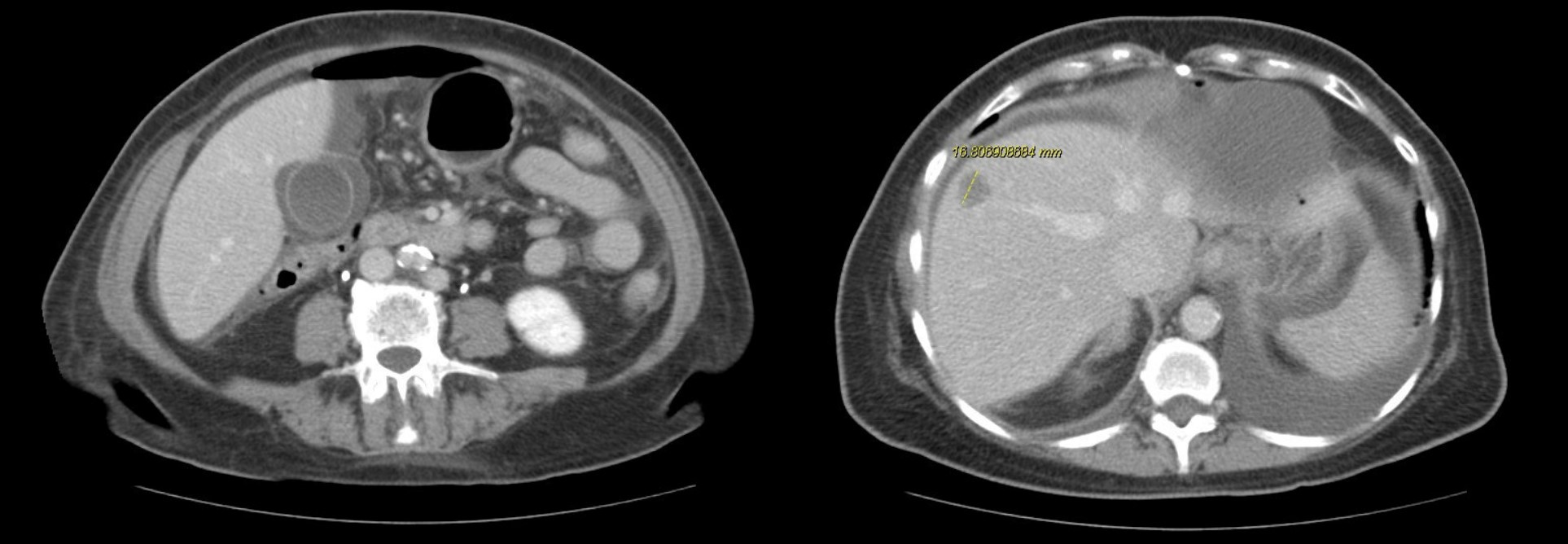
CT abdomen/pelvis shows free intraperitoneal air, small to moderate-sized volume of free intraperitoneal fluid, small low-density areas within enlarged liver concerning for metastases, large pericardial effusion, small to moderate-sized bilateral pleural effusions, nodular opacities at both lower lung zones concerning for metastatic involvement. (Figure 2)
Figure 2. CT demonstrating free intra-abdominal fluid and air, liver mass, and pleural effusions.
Discussion
This patient presented with worsening right-sided abdominal pain without fever but a concerning abdominal exam. Labs demonstrated elevated lactate, transaminitis, and elevated alkaline phosphatase, but no leukocytosis or elevation in bilirubin or lipase. RUQ ultrasound showed a dilated CBD but did not show any stones, gallbladder wall thickening, or wall edema. Free fluid was also visualized, increasing concern for viscus perforation or other abdominal process, and a CT scan was ordered which demonstrated free air and evidence of metastatic disease with hepatic and pulmonary involvement as well as a large pericardial effusion. The patient was taken for exploratory laparotomy which demonstrated a perforated duodenal ulcer.
The patient had multiple abnormal findings and labs, which were not initially consistent with a single diagnosis. Her absence of leukocytosis afebrile presentation could have been secondary to early stages of her perforation or possibly an immunocompromised state, while her elevated lactate could have been secondary to hypoperfusion in the setting of her sepsis or pericardial effusion. Her transaminitis was likely secondary to metastatic involvement of her liver in the absence of biliary pathology. In the setting of multiple potential medical problems, clinical data that may at first appear confounding needs to be carefully considered.
Conclusion
Consider alternative diagnoses to cholecystitis when a patient presents with RUQ pain & Murphy’s sign but no gallbladder wall thickening or edema on ultrasound.
POCUS, on the Rocks!
Scott Bickel, DO and Monika Lusiak, MD
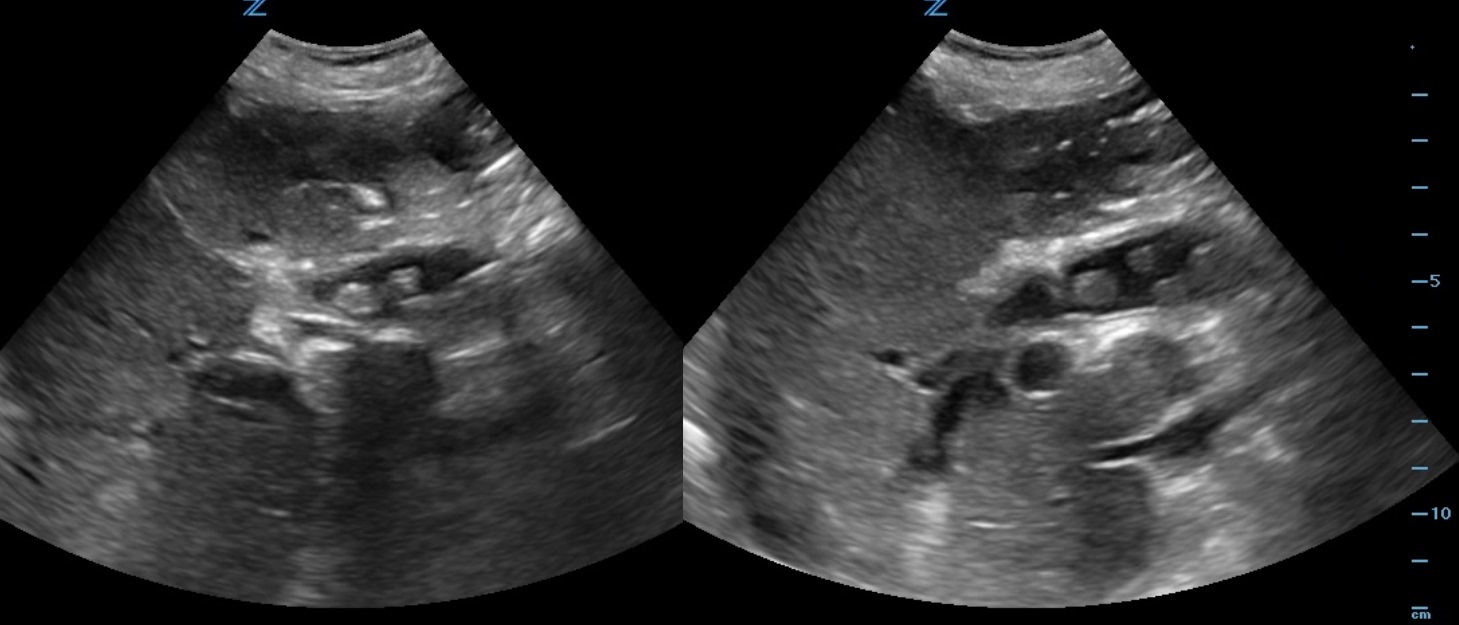 |
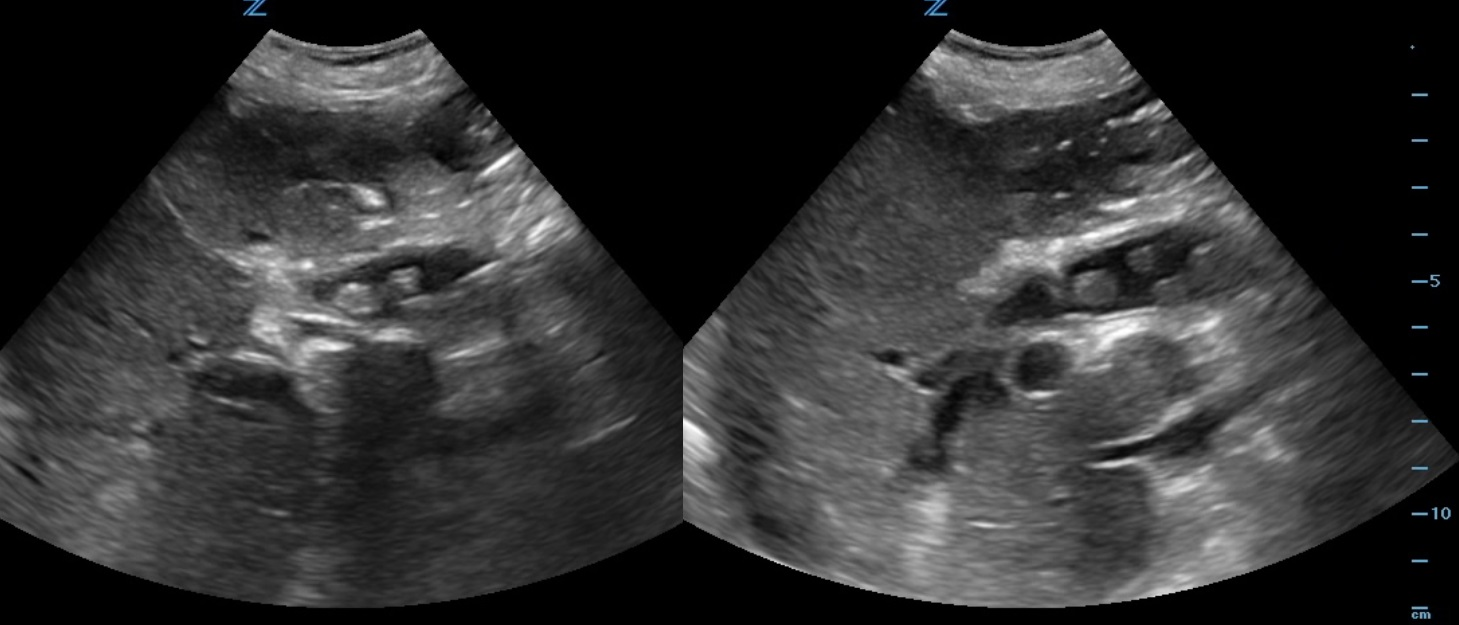 |
Figure 1-2. Longitudinal ultrasound of the RUQ.
Case Introduction
A 90-year-old female, with history of prior cholecystectomy, presents to the ED from the nursing home for a “leukocytosis of 18.” She complains of some lower abdominal pain, which has been ongoing for “years.” States she feels nauseated but denies emesis. She is unable to describe her symptoms any further. Denies fever or any other complaints. She is afebrile and her vital signs are within normal limits. On physical exam, she is in no distress, anicteric, and her abdomen is soft, non-tender, and non-distended. Labs are significant for a WBC count of 20.0k/mm3, Lipase 3 IU/L, Total bilirubin 3.6 mg/dL, Direct bilirubin 2.79 mg/dL, ALP 303 IU/L, AST 53 IU/L, ALT 21 IU/L, Na 126 mmol/L, Cr 2.19 mg/dL. Her chest X-ray shows no acute findings. A bedside right upper quadrant ultrasound is performed (Fig 1-2).
Case Discussion
The CT abd/pelvis for this patient confirmed multiple CBD stones including a 1.6cm obstructing stone. The patient was started on IV Piperacillin / Tazobactam and she was admitted for acute cholangitis with GI and ID consults. Primary choledocholithiasis is the formation of stones within the common bile duct (CBD), with an absent gallbladder. cystic fibrosis, recurrent biliary infections, East Asians, and elderly. Diagnosis is made by clinical suspicion, direct visualization on US, clinical cholangitis, bilirubin 1.8-4 mg/dL or greater, CBD >6 mm, age >55, LFT abnormalities and clinical gallstone pancreatitis. The ED management is focused on obtaining GI or surgery consultation and treating the complications.
Questions
- What is the diagnosis based on the photo?
- What is your differential diagnosis?
- What are the two most common complications?
Answers
- Primary Choledocholithiasis
- Differential diagnoses include cholecystitis, cholangitis, hepatitis, cholangiocarcinoma, pancreatic cancer, lymphoma, sarcoidosis, tuberculosis, and iatrogenic strictures
- Acute cholangitis and pancreatitis
Clinical Pearls
- Choledocholithiasis risk has been estimated at 5-20% at the time of cholecystectomy, with incidence increasing with age.
- Diagnosis made by clinical suspicion, US imaging, LFTs, CBC, Lipase, and CT if indicated.
- ED management focused on obtaining GI or Surgery consults and recognizing/treating any complications, namely, acute cholangitis or pancreatitis.
References
- Mustafa A Arain, MD, Martin L Freeman, MD, Nabeel Azeem, MD. Choledocholithiasis: Clinical manifestations, diagnosis, and management. UpToDate. Waltham, MA: UpToDate Inc. https://www.uptodate.com. (Accessed 9/24/19).
- Yang MH, Chen TH, Wang SE, et al. Biochemical predictors for absence of common bile duct stones in patients undergoing laparoscopic cholecystectomy. Surg Endosc 2008; 22:1620.
- Gurusamy KS, Giljaca V, Takwoingi Y, et al. Ultrasound versus liver function tests for diagnosis of common bile duct stones. Cochrane Database Syst Rev 2015; :CD011548.
Resident Section
Retrobulbar Hematoma
Alyssa Alloy, MD and Neeraj Gupta, MD FAAEM
Case Presentation
This is a 63-year-old man with a history of alcohol abuse who presented to the emergency department after an assault. He was allegedly hit in the face with the blunt end of a pistol. Examination revealed a large periorbital hematoma with significant swelling limiting his ability to open his eye. Initial ocular exam was extremely limited. A bedside ocular ultrasound demonstrated a hypoechoic collection posterior to the globe concerning for retrobulbar hematoma.
The patient urgently underwent a CT of the facial bones, which demonstrated extensive fractures throughout the orbit, maxilla, and nasal bones and confirmed the presence of intraorbital, extraconal and retrobulbar hematomas with proptosis. Ophthalmology and ENT were consulted given the concern for orbital compartment syndrome. A bedside right lateral canthotomy and cantholysis was performed to relieve pressures. Post procedure, the patient was admitted to the Trauma service for ongoing monitoring and taken to the OR with ENT the following day for open reduction and internal fixation of the right orbital floor.
Discussion
Facial trauma to the eye can notably cause orbital fracture and subsequent retrobulbar hematoma. This is an ocular emergency, which can lead to orbital compartment syndrome and vision loss by sudden increase in IOP resulting in ischemia of the optic nerve. Rapid decompression with lateral canthotomy and cantholysis is crucial to preserve vision. Retrobulbar hematoma is a clinical diagnosis that can be made by identifying restricted EOM, painful proptosis with resistance to retropulsion. There may also be an afferent pupillary defect, and swollen or pale optic disc. However, exams are frequently limited in a traumatic setting. A point-of-care ocular ultrasound can rapidly detect for ocular trauma when physical exam can’t be performed reliably. A linear array probe is placed over the closed eye, being careful to avoid applying pressure to the eye. A tegaderm can be used. Retrobulbar hematoma may appear as anechoic or hyperechoic within the hyperechoic retrobulbar area just posterior to the globe. It is important to note that ocular ultrasound is relatively contraindicated if there is globe rupture. Though diagnosis may be confirmed by CT, bedside ultrasound may be used to supplement CT if imaging is unavailable or delayed, as time to decompression in compartment syndrome is critical in order to prevent vision loss.
References
- Kniess, C. K., Fong, T. C., Reilly, A. J., & Laoteppitaks, C. (2015). Early Detection of Traumatic Retrobulbar Hemorrhage Using Bedside Ocular Ultrasound. The Journal of Emergency Medicine, 49(1), 58-60. doi:10.1016/j.jemermed.2014.12.074
- Leshner, M., Gibbons, R., & Costantino, T. (2018). Adult Male with Traumatic Eye Pain and Swelling. Clinical practice and cases in emergency medicine, 2(2), 169–170. https://doi.org/10.5811/cpcem.2018.2.36857
- Tintinalli, J. E., Stapczynski, J. S., Ma, O. J., Yealy, D. M., Meckler, G. D., & Cline, D. (2016). Tintinalli's emergency medicine: A comprehensive study guide (Eighth edition.). New York: McGraw-Hill Education.
Ultrasound Highlights
Massive PE and More
Joseph Pinizzotto, DO; Meghan Tape, DO; Jonathan Kaplan, MD; and Neeraj Gupta, MD FAAEM
A 67-year-old woman with a past medical history of Atrial fibrillation and COPD presented to our emergency department with acute hypoxic respiratory failure and hypotension. She required emergent endotracheal intubation and was started on norepinephrine for blood pressure support. Following initial stabilization, a focused cardiac ultrasound (FOCUS) was obtained to evaluate for potential causes of shock. Ultrasound findings revealed dilatation of the right atrium and right ventricle. There was a significantly reduced right ventricular systolic function and global hypokinesis with apical sparing consistent with McConnell’s sign. In addition, a free-floating thrombus in transit was visualized in the right atrium and right ventricle. After stabilization, a CT angiography of the chest was obtained which confirmed the presumptive diagnosis showing extensive pulmonary emboli with signs of right heart strain. Cardiothoracic surgery was consulted, Heparin infusion was initiated, and the patient was promptly taken to the operating room for surgical thrombo-embolectomy. Our images show the importance of obtaining a FOCUS for all undifferentiated shock patients. This rapid, bedside tool narrowed the differential, expedited advanced imaging, and led to a timely disposition.
The Aortic Flap Dance
Alyssa Alloy, MD and Neeraj Gupta, MD FAAEM
We present a 58-year-old man with a history of hypertension who presented to our emergency department with an abrupt onset of chest, back and abdominal pain. He reported severe central chest pain with radiation to the back. He was significantly hypertensive with a systolic blood pressure >200 mmHg. There was immediate concern for acute aortic pathology. A point-of-care aorta ultrasound was performed. The images showed a clear dissection intimal flap in the abdominal aorta. On ultrasound, this appears as a hyperechoic linear structure within the aortic lumen.
Our patient was immediately initiated on antihypertensive medications and received a CT of the chest, abdomen, and pelvis with IV contrast which confirmed a Stanford Type B dissection originating just distal to the left subclavian artery and extending down to the iliac artery. He was admitted to the SICU for continued medical management.
Although not sensitive, point-of-care ultrasound is highly specific for the diagnosis of aortic dissection.3 Our case illustrates the importance of timely POCUS in expediting diagnosis and treatment of this critical disease process.
References
- Colla, J. S., MD. (2017). Emergency Ultrasound: Identification of Aortic Dissection Using Limited Bedside Ultrasound. Emergency Medicine. doi:10.12788/emed.2017.0017
- Kehrl, T., & Ball, L. (2017, February 01). Can't Miss Killer: The Role of Point-of-Care Ultrasound in Diagnosing Acute Aortic Dissection. Retrieved September 18, 2020, from https://www.emra.org/emresident/article/cant-miss-killer-the-role-of-point-of-care-ultrasound-in-diagnosing-acute-aortic-dissection
- Wang Y, Yu H, Cao Y, Wan Z. Early Screening for Aortic Dissection With Point-of-Care Ultrasound by Emergency Physicians: A Prospective Pilot Study. J Ultrasound Med. 2020 Jul;39(7):1309-1315. doi: 10.1002/jum.15223. Epub 2020 Jan 23. PMID: 31971274.
Always Grateful for a Water Bath
Joseph Ciano, DO
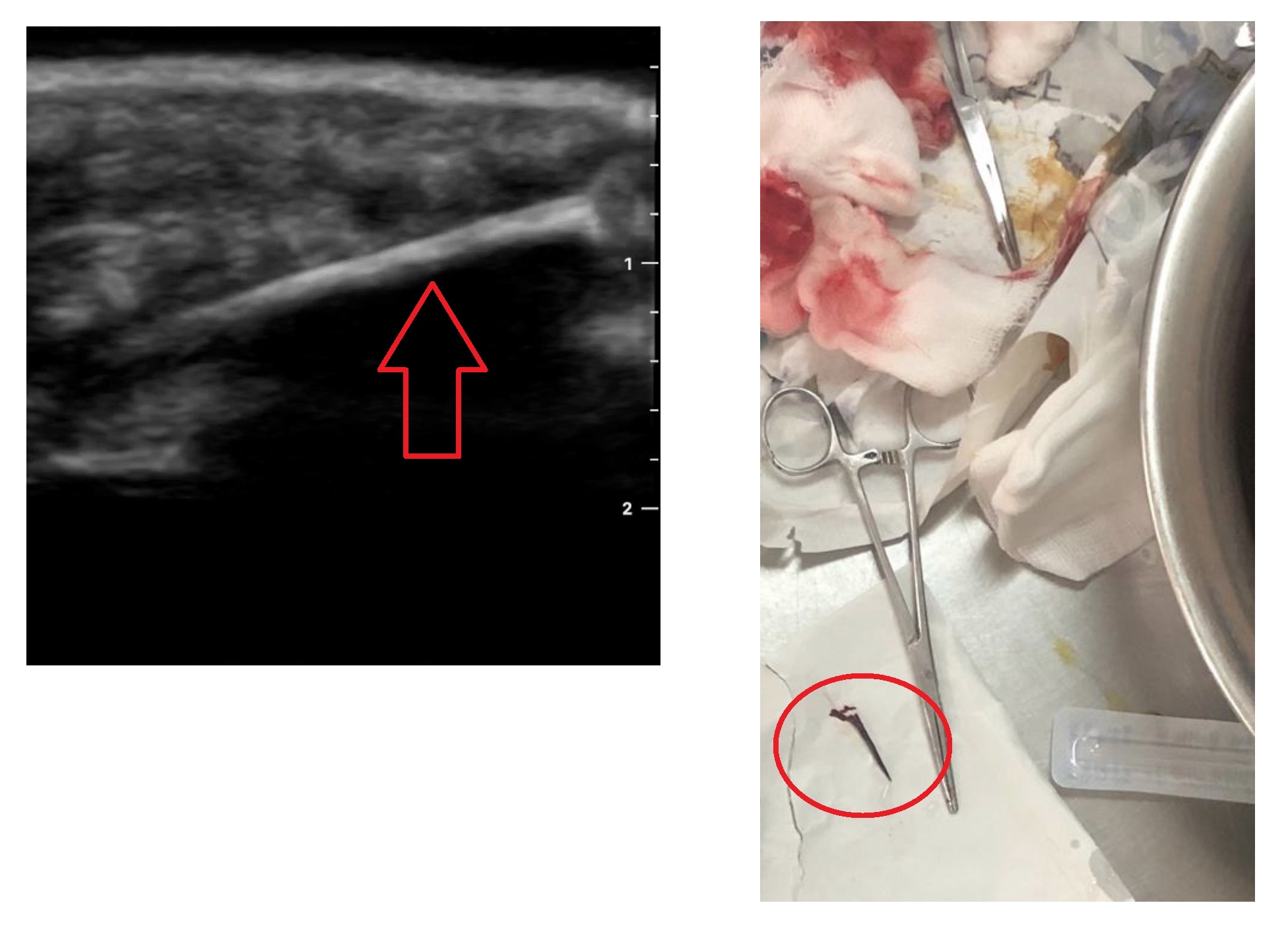
A young male presented to the emergency department of a public sector regional referral hospital in Uganda. His reason for visiting the hospital was for evaluation of a retained foreign body in the thenar eminence area of the left hand. The foreign body was thought to be a piece of wood, a long thorn, or some other plant matter. Removal of the foreign body without ultrasound was difficult due to poor visualization of the tract of entry and depth of penetration. Point-of-care ultrasound (POCUS) in combination with a water bath was used to identify the size, location, and depth of the object in order to facilitate its removal. The object was successfully removed under ultrasound guidance with an approximate measurement of 3 cm.
Fellow Section
An Unusual Case of Adult Intussusception
Dylan Smith, MD and Shawn Sethi, DO
A 60-year-old man presented to the emergency department with a chief complaint of abdominal pain. He described a day and a half of lower abdominal pain that was progressive in nature and described as gnawing. He noted associated mild anorexia and nausea without emesis. His last bowel movement was one day ago, was reportedly normal and he had been passing flatus. He took over-the-counter antacids and analgesics without any relief. He had no pertinent past medical history. His surgical history was notable for a right umbilical hernia repair 10 years prior.
On examination, his vitals were within normal limits except for mild hypertension. He appeared to be a well-nourished, otherwise well-appearing male of stated age. His abdominal exam was notable for tenderness with mild guarding in the periumbilical, suprapubic, and right lower quadrants. There were no palpable masses and he had no evidence of hernia defects on palpation.
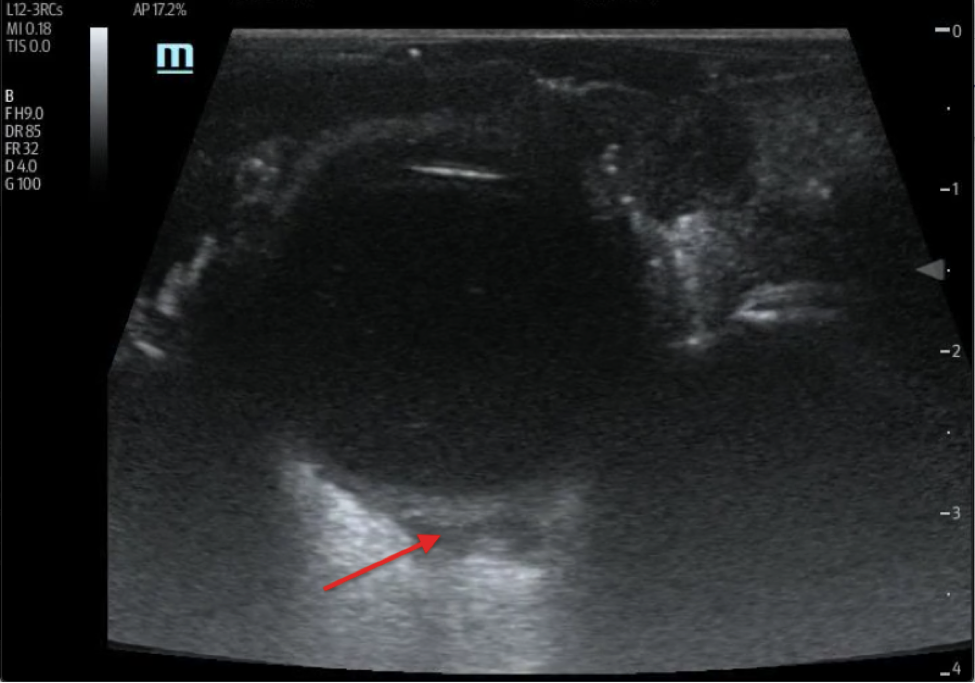
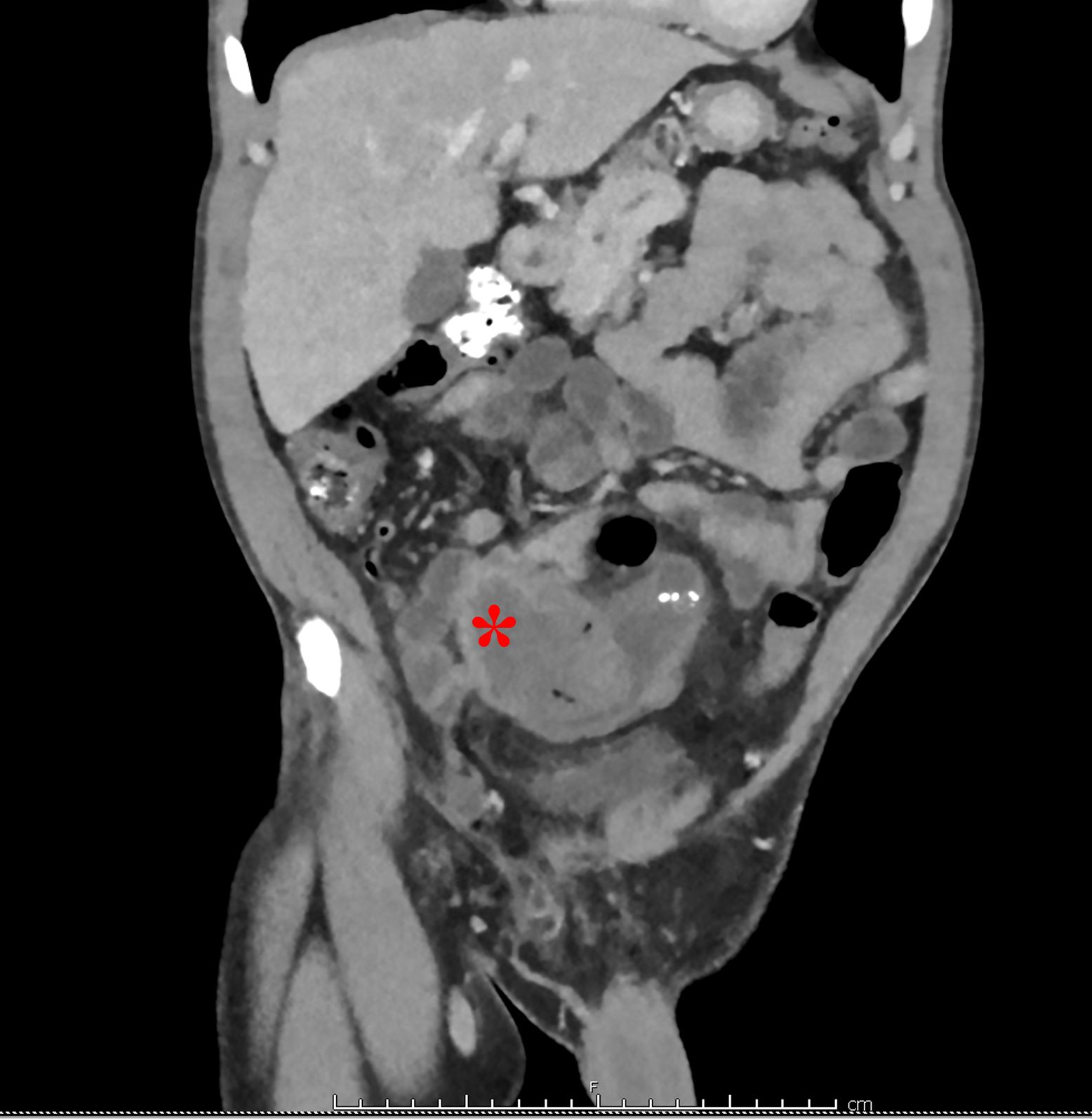
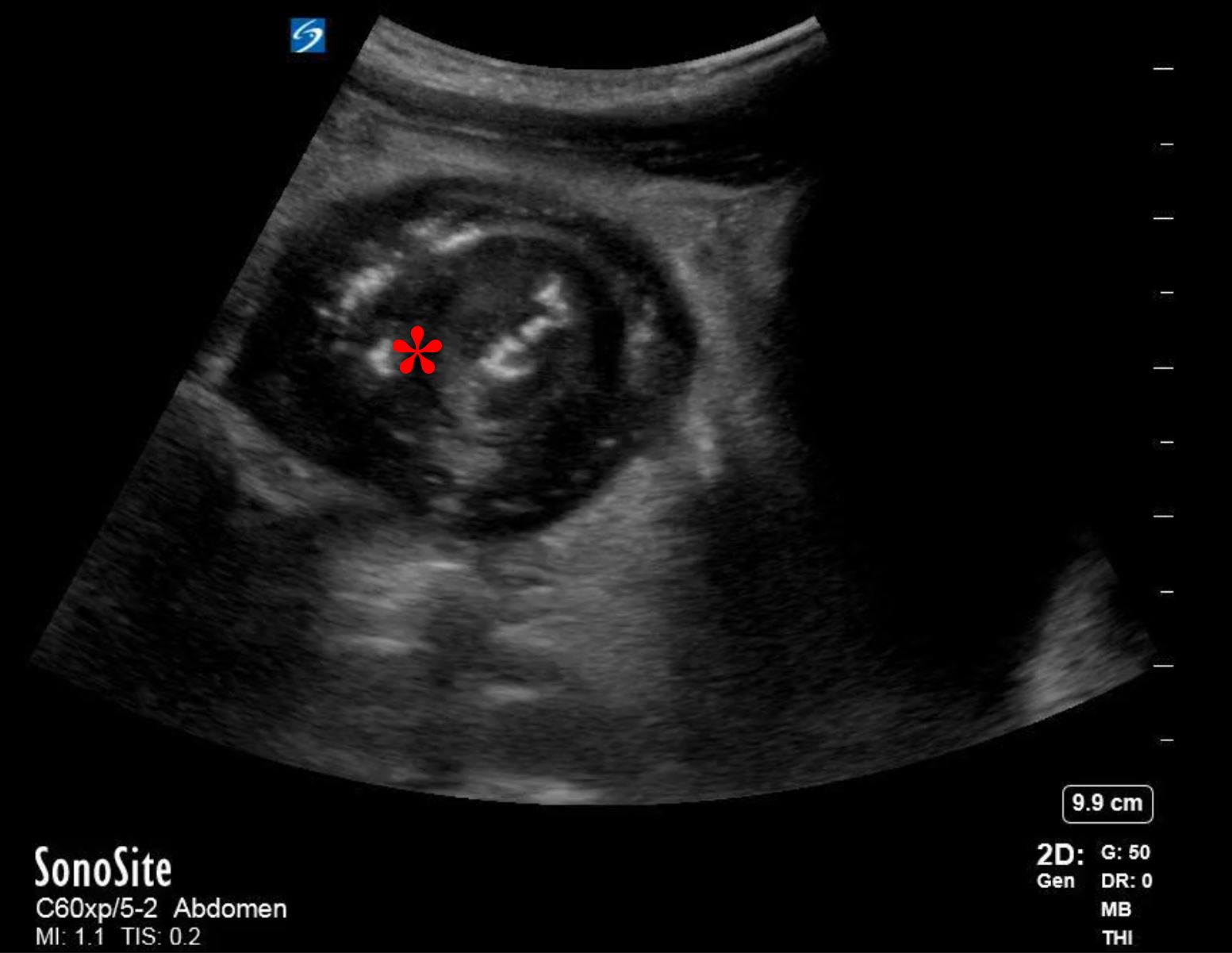
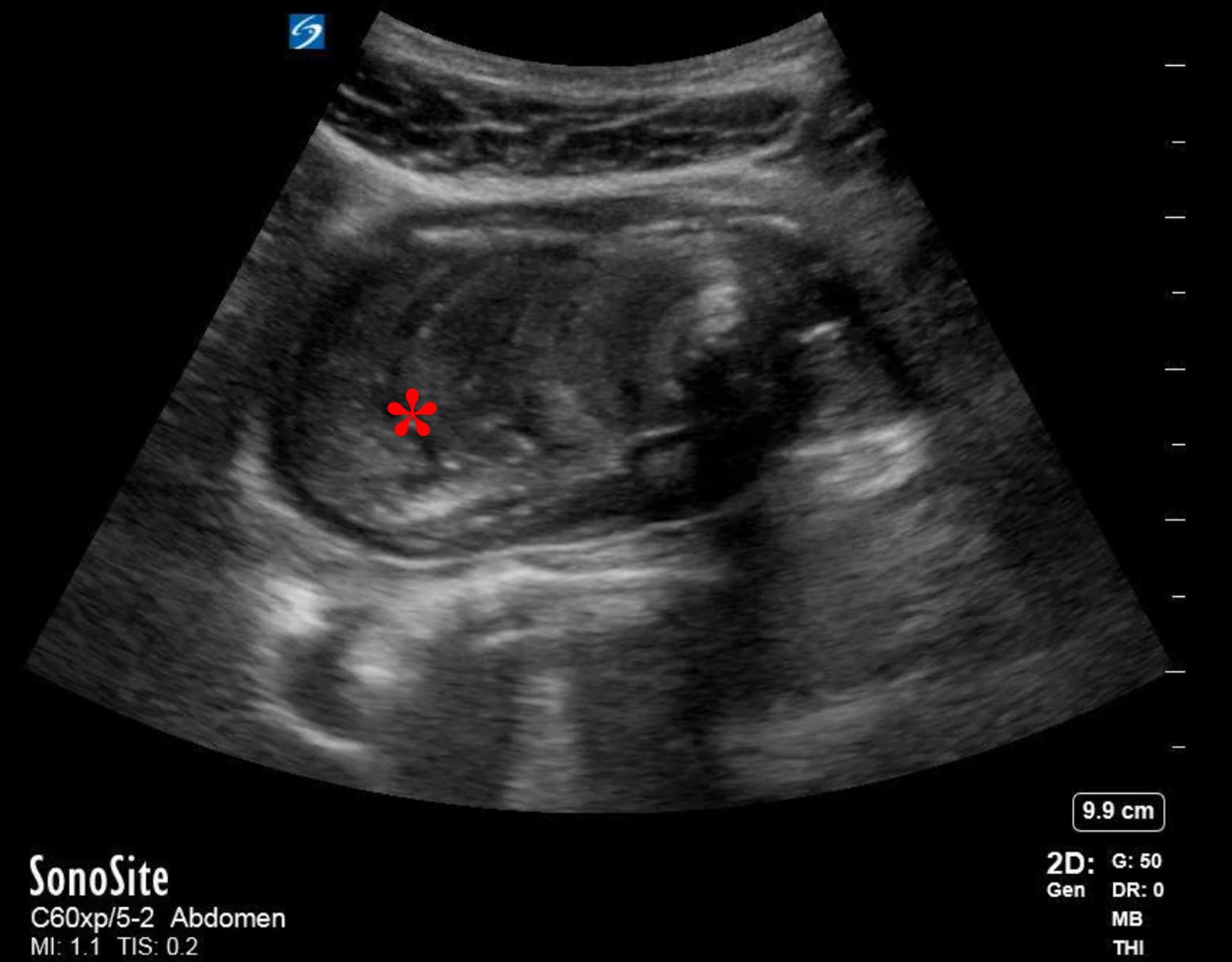
His bloodwork showed multiple abnormalities including a leukocytosis with a left-shift and an elevated lactic acid. His CT scan showed a focally dilated and abnormal appearing loop of small bowel in the middle of the abdomen (Figure 1). On discussion with radiology, the patient is suspected to have ileo-ileal intussusception without an identifiable mass or lead point. A bedside ultrasound is performed which showed a focally dilated loop of small bowel with bowel wall thickening, abnormal peristalsis, and a “target” sign (Figure 2).
General surgery admitted the patient for laparotomy. On hospital day two he had an open laparotomy and was found to have intussusception secondary to a perforated Meckel’s diverticulum. He underwent small bowel resection with anastomosis. Post-operatively the patient did well and was discharged home on hospital day seven.
Discussion
This patient presented with two interconnected pathologies, which are more frequently seen in pediatric patients. Intussusception is classically considered a disease of early childhood, with an incidence of 50-100 cases in pediatric patients per 100,000 live births.1 The majority of these cases develop before the age of three. In children, the commonly considered presentation involves waxing and waning abdominal pain with constipation and eventual currant jelly stools. Adults account for fewer than 5% of all cases of intussusception and, among adults presenting with small bowel obstruction, it is found to be the cause in 1-5% of cases.2 The presentation of intussusception in adults is typically similar to that of other small bowel obstructions. Surgery is the standard of care for adult intussusception, rather than endoscopic management or air enema, due to the high rate of malignant lesions as the cause. Up to 30% of adult intussusceptions caused by a mass are due to malignancy.3
Meckel’s diverticulum is the most common congenital malformation of the GI tract, occurring in approximately 2% of the population.8 Symptomatic Meckel’s diverticula are also more frequently encountered in the pediatric population. In one study, over half of all patients presenting with symptoms due to complications from Meckel’s were under the age of 20.8 Typical presenting symptoms vary but include hematochezia and melena, intermittent abdominal pain, recurrent small bowel obstruction, and intussusception. The majority of cases of Meckel’s diverticulum remain asymptomatic throughout life and do not require surgery if found incidentally.9
Point-of-care ultrasound (POCUS) can be used for the diagnosis of intussusception. Using a linear or curvilinear transducer (depending on the patient’s body habitus) in the transverse view, the area of interest will appear as a hypoechoic ring, representing the edematous bowel walls, around an echo-dense center, representing the mucosal and serosal layers of the trapped bowel. This is also known as the “bulls-eye” or “target” sign. In the longitudinal view there will be appearance of three parallel hypoechoic areas separated by hyperechoic zones, also known as the “hayfork” or “sandwich” sign which represent telescoping of bowel.4
Potential false positives on ultrasound include colitis, hematoma or mass. False negatives may include incomplete visualization of the area secondary to bowel gas. Using a lawn-mower technique and graded compression along the scanning path can improve views and accuracy.
Use of POCUS for diagnosing intussusception compared to CT scan carries several advantages including a lack of ionizing radiation, non-invasive nature, and ability to reliably repeat the study on the same patient over time.
Although traditionally ultrasound for intussusception is performed and interpreted by radiologists, there is a growing literature to support bedside diagnosis by emergency providers. In a prospective observational study of 82 pediatric patients, pediatric emergency medicine physicians were able to diagnose ileo-colic intussusception by POCUS with a sensitivity of 85% and specificity of 97% after a 1-hour training session.5 In addition, a recent systematic review and meta-analysis of 1,303 children found that POCUS performed by emergency physicians for intussusception was 94.9% sensitive and 99.1% specific.6 Although diagnosis of intussusception by ultrasound is typically made in pediatric patients, it can be used in adults as seen in our patient. Case reports have shown the use of contrast enhanced ultrasound for diagnosis in adult patients, but more research is needed.7
Figures
Figure 1. Coronal CT showing distended small bowel in the distal ileum at the site of intussusception (Denoted with *)
Figure 2A. Transabdominal transverse view ultrasound showing distended small bowel with classic “target” or “bulls-eye” sign (Denoted with *)
Figure 2B. Transabdominal longitudinal view ultrasound showing distended small bowel with the “sandwich” sign related to the telescoped small bowel (Denoted with *)
References
- Buettcher, M., Baer, G., Bonhoeffer, J., Schaad, U.B., Heininger, U. Three-year surveillance of intussusception in children in Switzerland. Pediatrics. 2007;120(3):473-480.
- Marinis, A., Yiallourou, A., Samanides, L., Dafnios, N., Anastasopoulos, G., Vassiliou, I., & Theodosopoulos, T. (2009). Intussusception of the bowel in adults: a review. World journal of gastroenterology, 15(4), 407–411
- Zubaidi, A., Al-Saif, F., & Silverman, R. (2006). Adult intussusception: a retrospective review. Diseases of the colon and rectum, 49(10), 1546–1551.
- Ramsey, K. W., & Halm, B. M. (2014). Diagnosis of intussusception using bedside ultrasound by a pediatric resident in the emergency department. Hawai'i journal of medicine & public health, 73(2), 58–60.
- Riera, A., Hsiao, A. L., Langhan, M. L., Goodman, T. R., & Chen, L. (2012). Diagnosis of intussusception by physician novice sonographers in the emergency department. Annals of Emergency Medicine, 60(3), 264–268.
- Lin-Martore M, Kornblith AE, Kohn MA, Gottlieb M. (2020). Diagnostic Accuracy of Point-of-Care Ultrasound for Intussusception in Children Presenting to the Emergency Department: A Systematic Review and Meta-analysis. West J Emerg Med. 21(4):1008-1016.
- Rafailidis, V., Phillips, C., Yusuf, G., & Sidhu, P. (2017). A case of adult intussusception with greyscale, contrast-enhanced ultrasound and computerised tomography correlation. Ultrasound, 25(2), 120–125.
- Park, J. J., Wolff, B. G., Tollefson, M. K., Walsh, E. E., & Larson, D. R. (2005). Meckel diverticulum: the Mayo Clinic experience with 1476 patients (1950-2002). Annals of surgery, 241(3), 529–533.
- Zani, A., Eaton, S., Rees, C. M., & Pierro, A. (2008). Incidentally detected Meckel diverticulum: to resect or not to resect?. Annals of surgery, 247(2), 276–281.
US Guided Procedures
Ultrasound Guided Nerve Block of the Posterior Tibial Nerve
Jiodany Perez, MD and Michael Rosselli, MD FAAEM
Introduction
Injuries sustained to the sole of the foot are a common presentation in the emergency department. At times, it may be necessary to perform procedures related to these injuries; including laceration repair, wound exploration and foreign body retrieval. Achieving good anesthesia to this area is crucial when performing these procedures. Direct cutaneous injection of a local anesthetic to the sole of the foot has traditionally been used to achieve anesthesia to this area. However, this method is extremely painful for patients making the required procedure more difficult for both the patient and the clinician alike.
Peripheral nerve blocks have been used to achieve anesthesia to difficult areas, like the sole of the foot, where local cutaneous injections proved to be poorly effective or difficult to perform. The Posterior Tibial Nerve (PTN), a branch of the sciatic nerve (L3-S4), is responsible for providing sensory innervation to the sole of the foot. Achieving a successful block of the PTN can dramatically change the dynamic of procedures required in this difficult area.
Blind (anatomic landmark guided) blocks of the PTN have been used to achieve anesthesia to the sole of the foot. However, complete block success rates using the blind approach have been documented to be as low as 22%. One of the more recent applications of POCUS (point-of-care ultrasound) utilizes needle guidance to achieve successful regional anesthesia. A recent study published in the Journal of Regional Anesthesia and Pain Management demonstrated increased success rates, decreased amount of required supplemental opioid use and decreased amount of unplanned general anesthesia when comparing anatomic landmark guided techniques vs ultrasound guided PTN blocks. In this article, we will describe a simple technique to perform a successful ultrasound guided nerve block of the PTN.
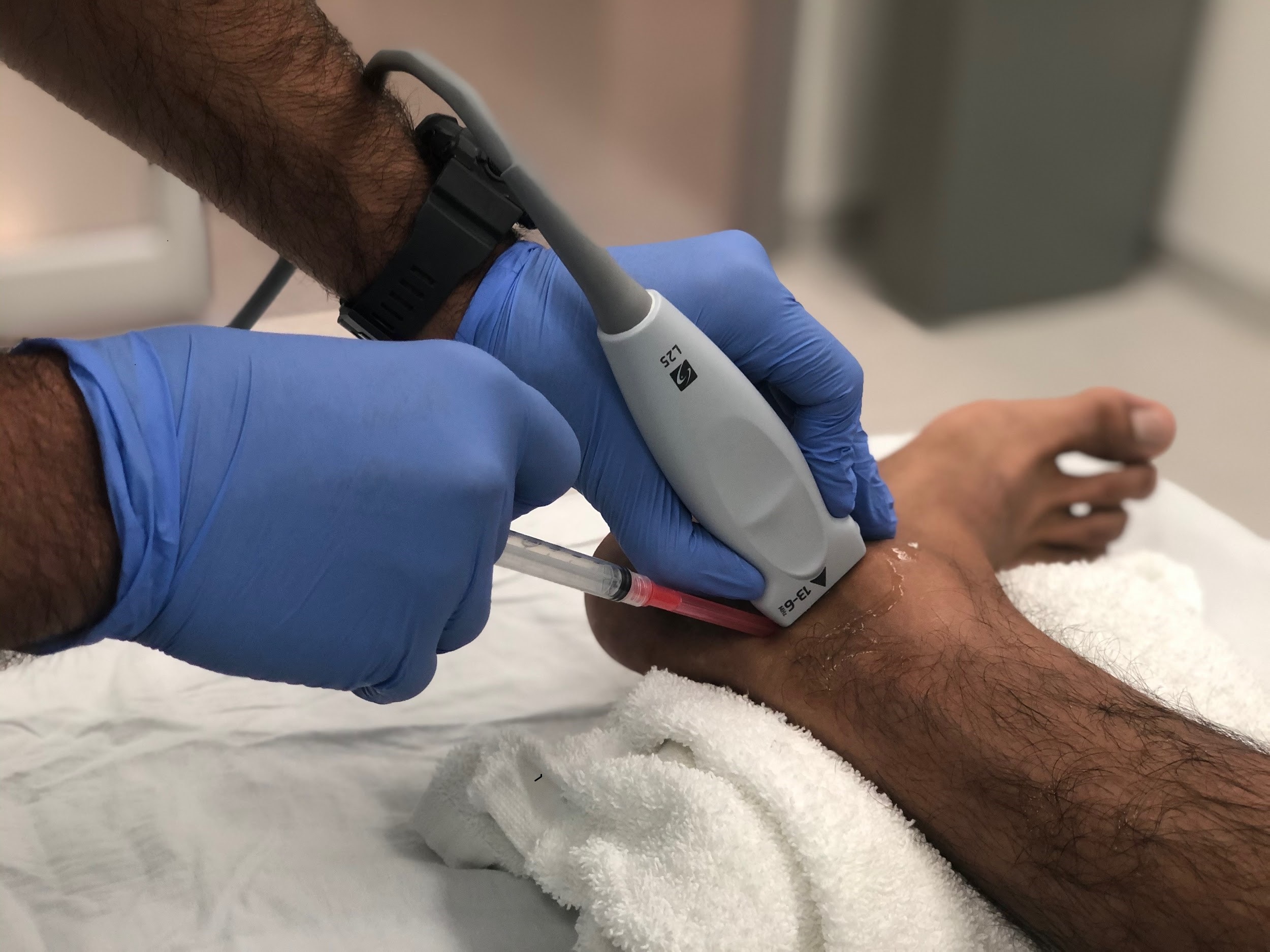
Scanning technique
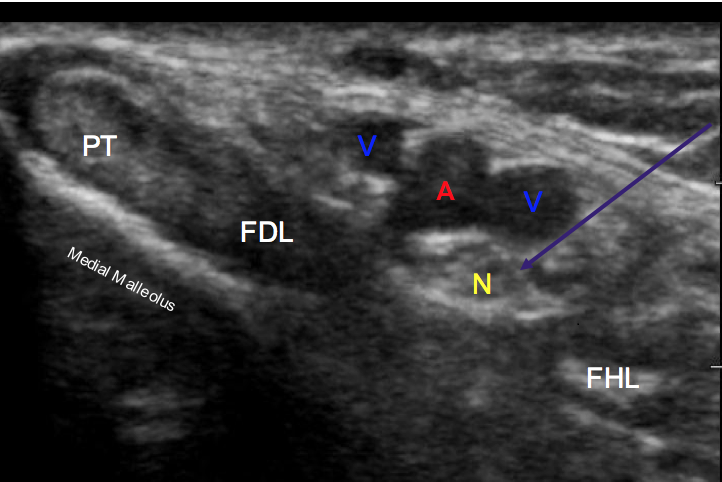
Using a linear array probe (high frequency 13-6 MHZ) placed just posterior to the medial malleolus in the short axis orientation as demonstrated in Figure 1, search for the tibialis posterior (TP) tendon. Just posterior to the TP tendon you will find the tendon of the flexor digitorum longus (FDL) and slightly posterior to this structure you will find the neurovascular bundle. This bundle includes the posterior tibial nerve, posterior tibial artery and the posterior tibial veins. The posterior tibial nerve can be differentiated by the hyperechoic “honeycomb” appearance of the nerve compared to the pulsatile hypoechoic nature of the artery and the nonpulsatile hypoechoic appearance of the veins (Figure 2).
Procedure
Once the appropriate structures are identified, insert a 4-5 cm 25 gauge needle in plane at a 30-45 degree angle (Figure 1). Locate the needle tip and advance the needle until you approach the nerve sheath (Figure 2). Slowly inject 5-10 cc of local anesthetic while advancing the needle tip adjacent to the nerve so that the anesthetic completely surrounds the PTN. Adequate regional anesthesia should be achieved within 15 minutes of local anesthetic injection.
Images
Figure 1. Probe and needle position for in plane Ultrasound guided posterior nerve block.
Figure 2. Posterior Tibial nerve block in plane view. Purple arrow: Needle path; Yellow “N”: Posterior tibial nerve; Red “A”: Posterior tibial artery; Blue “V”: Posterior tibial vein; PT: Tibialis Posterior tendon; FDL: Flexor Digitorum Longus; FHL: Flexor Hallucis Longus.
References
- Joseph Chorley, AR (2013). Forefoot and midfoot pain in the active child or skeletally immature adolescent: Overview of causes. In UpToDate, A. Hergenroeder, & R. Baucher (Eds), Available from: https://www.uptodate.com/contents/forefoot-and-midfoot-pain-in-the-active-child-or-skeletally-immature-adolescent-overview-of-causes?
- Redborg, KE Ultrasound Improves the Success Rate of a Tibial Nerve Block at the Ankle. Reg Anesth Pain Med. 2009: 34(3):256-60.
- Chin, K Ultrasound-guided Versus Anatomic Landmark-Guided Ankle Blocks: A 6 year Retrospective Review. Reg Anesth Pain Med. 2011; 36(6):611-8.
- Strakowski, J. Ultrasound-Guided Peripheral Nerve Procedures. Phys Med Rehabil Clin N Am. 2016 Aug; 27(3):687-715.
- Vandepitte C, Lopez A, Van Boxstael S, Jalil H. Ultrasound-Guided Nerve Block. NYSORA. 2020. Available from: https://www.nysora.com/regional-anesthesia-for-specific-surgical-procedures/lower-extremity-regional-anesthesia-for-specific-surgical-procedures/foot-and-anckle/ultrasound-guided-ankle-block/
Traumatic Knee Joint Aspiration
Sara Zagroba, MD and Michael Rosselli, MD FAAEM
Case
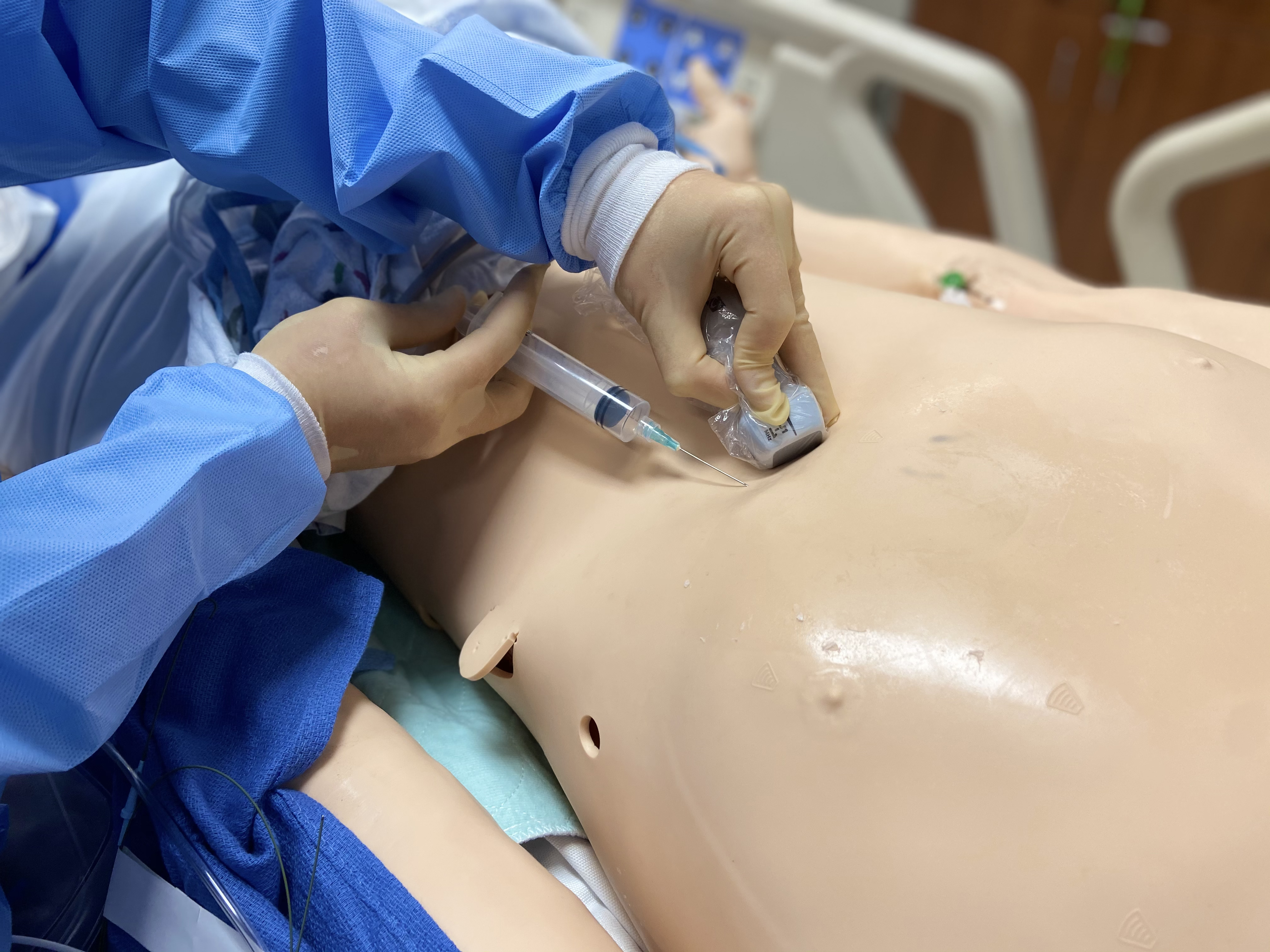
A 36-year-old male presented to the emergency department with a chief complaint of right knee pain status post motor vehicle accident that occurred just prior to arrival. The patient was a restrained driver, no air bag deployment, involved in a low speed motor vehicle accident. Patient states that he hit his right knee on the dashboard and is now complaining of worsening severe right knee pain associated with difficulty ambulating. Physical exam revealed a knee effusion, diffuse tenderness and severely limited flexion of the right knee. A knee X-ray was obtained and revealed no fracture or dislocation. A point-of-care ultrasound of the right knee showed a large anechoic joint effusion with multiple hyperechoic shadows in the supra-patellar recess. Prior to discharge, an ultrasound-guided knee joint aspiration with lidocaine was performed as described below.
Introduction
Acute knee pain is a common complaint in the emergency department (ED) and is one of the most common musculoskeletal symptomatic sites.1,2 Common mechanisms can be from direct contact trauma, such as a fall or a motor vehicle crash, or from non-contact trauma, such as running, jumping, or twisting-motion. The initial workup includes a knee X-ray to identify a fracture and/or dislocation. Frequently, there is no underlying fracture or dislocation, and yet the patient has significant knee swelling and pain. In these cases, acute knee pain is often secondary to ligament or tendon injury, which requires further imaging with an MRI, not performed routinely in the ED. A musculoskeletal point-of-care ultrasound (POCUS) allows for an emergency medicine physician to rapidly assess the joint with real-time images with sensitivities and specificities approaching 100% and make appropriate medical decisions, including arthrocentesis.3
Knee effusion in the setting of acute traumatic knee pain is usually secondary to blood product accumulation in the joint space from significant structural damage. The accumulation of blood in the knee joint produces additional pressure, resulting in pain and decreased range of motion of the knee, especially with knee flexion. By utilizing ultrasound-guided knee aspirations in the acute traumatic knee effusion, the clinician will be able to provide the patient with additional pain relief prior to disposition from the emergency department.4,5 In comparison to standard landmark technique for knee arthrocentesis, ultrasound-guided knee arthrocentesis offers greater accuracy and clinical improvement, with reduced number of attempts and decreased patient-reported pain, respectively.6 Ultrasound-guided aspiration also has been shown to maximize the amount of fluid evacuated compared to the standard landmark technique.6 Thus, ultrasound-guided aspirations should be used for both diagnostic and therapeutic purposes in patients who present with traumatic knee pain and effusion in the emergency department.
Scanning Technique7
Blood and synovial fluid often collect in the suprapatellar recess. Thus, the ideal location of ultrasound-guided aspiration is via the superolateral approach. To evaluate the suprapatellar recess for fluid, the patient’s knee is placed with approximately 30 degrees of knee flexion. The knee flexion distends the suprapatellar recess between the quadriceps tendon and the prefemoral fat pad, which allows for the optimal view of synovial fluid and/or blood products. Additionally, detection of fluid in the suprapatellar recess may be improved with quadriceps contraction. To maximize the size of the suprapatellar joint recess and prevent displacement of joint fluid, transducer pressure should be minimized.
First scan the quadriceps tendon in the longitudinal view in order to locate the suprapatellar recess. To obtain the long axis of the quadriceps tendon, the linear probe is placed along the sagittal plane on the distal anterior aspect of the thigh with the transducer marker pointed superiorly. The axis of the probe is parallel to the femoral shaft and quadriceps tendon. The view of the long axis of the quadriceps tendon appears hyperechoic with longitudinal fibers of the muscle inserting on the superior aspect of the patella. To maximize the view, use the heel toe and fanning maneuver of the probe. Deep to the hyperechoic fibers of the muscle, evaluate for anechoic or hypoechoic joint fluid which would be detected between the quadriceps tendon and the prefemoral fat pad.
Obtain the suprapatellar recess in the transverse view by rotating the probe ninety-degrees from the longitudinal view of the quadriceps tendon with the probe marker to the patient’s right (Figure 1). Slide the transducer lateral to maximize your view. Measure the depth of the joint fluid from the ultrasound probe and mark the skin with a needle cap on the lateral aspect of the knee. Sterilize the skin with a skin anti-septic solution. Using the in-plane needle aspiration approach, place the needle tip at the spot marked on the skin and align the needle parallel to the transducer. Visualize the needle entering the skin while maintaining the needle parallel to the transducer (Figure 2). Once the tip of the needle has entered the skin, slowly inject lidocaine while guiding the needle into the suprapatellar recess. Visualize on the ultrasound screen the needle traversing the suprapatellar recess (Figure 3). Aspirate the fluid in the suprapatellar recess (Figure 4). When no further joint fluid can be aspirated, remove the needle and apply pressure to the site with gauze. Recommend ice therapy after the procedure.
For aspiration, it is recommended to use a 18-gauge needle with 3-5 cc of lidocaine 1% without epinephrine in a 20-milliliter syringe (Figure 5).
Images
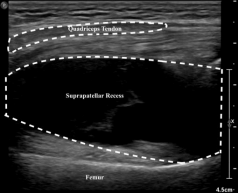
Figure 1. Transverse view of the suprapatellar recess.
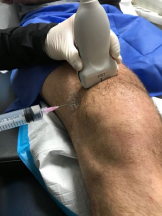
Figure 2. The in-plane needle aspiration approach. The needle tip at the spot marked on the skin based on the depth of the joint fluid from the ultrasound probe with the needle aligned parallel to the transducer.
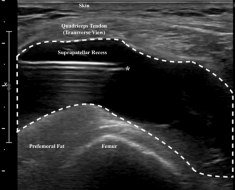
Figure 3. Transverse view of the suprapatellar recess with in-plane needle visualization. (*) denotes the needle tip within the suprapatellar recess.
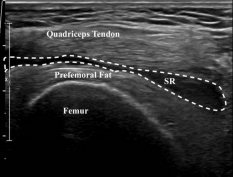
Figure 4. Transverse view of the suprapatellar recess post-aspiration. (SR) denotes collapsed suprapatellar recess post-aspiration of joint fluid.
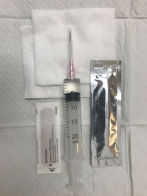
Figure 5. Supplies for aspiration: 18-gauge needle with 3-5 cc of lidocaine 1% without epinephrine in a 20 cc syringe.
References
- National Hospital Ambulatory Medical Care Survey. Emergency department summary tables. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2017_ed_web_tables 508.pdf; 2017.
- Situ-LaCasse E, Grieger RW, Crabbe S, Waterbrook AL, Friedman L, Adhikari S. Utility of point-of-care musculoskeletal ultrasound in the evaluation of emergency department musculoskeletal pathology. World J Emerg Med. 2018;9(4):262-266.doi:10.5847/wjem.j.1920-8642.2018.04.004
- Wu TS, Roque PJ, Green J, Drachman D, Khor KN, Rosenberg M, et al. Bedside ultrasound evaluation of tendon injuries. Am J Emerg Med. 2012;30(8):1617-21.
- Aceves-Avila FJ, Delgadillo-Ruano MA, Ramos-Remus C, Gomez-Vargas A, Gutierrez Urena S (2003) The first descriptions of therapeutic arthrocentesis: a historical note. Rheumatology (Oxford) 42:180–183
- Bhavsar TB, Sibbitt WL, Band PA, Cabacungan RJ, Moore TS, Salayandia LC, Fields RA, Kettwich SK, Roldan LP, Suzanne Emil N, Fangtham M, Bankhurst AD. Improvement in diagnostic and therapeutic arthrocentesis via constant compression. Clin. Rheumatol. 2018 Aug;37(8):2251-2259.
- Wu T, Dong Y, Song HX, Fu Y, Li JH. Ultrasound-guided versus landmark in knee arthrocentesis: A systematic review. Semin Arthritis Rheum. 2016;45(5):627-32.
- Jacobson, Jon A.. Fundamentals of Musculoskeletal Ultrasound. United States, Elsevier, 2017.
Administrative Corner
System-Wide Clinical Ultrasound Committees
Alexis Salerno, MD FAAEM
Do you think you have an organized ultrasound department but would like more communication throughout your hospital or clinical group? It may be time to develop a System-Wide Clinical Ultrasound Committee (SWCUS).
A SWCUS interacts with individuals and/or groups of practitioners using Clinical Ultrasound (CUS) in a variety of practice environments. It helps to promote communication amongst providers, avoid duplication of services and promotes high standards across the institution in all settings. The responsibilities of the SWCUS can be organized into 5 areas: (1) education, (2) credentialing, (3) workflow solutions, (4) equipment purchases and (5) reimbursement.
Education
To be competent in CUS, physicians must be able to master image acquisition, interpretation and be able to integrate these into their medical decision-making. Therefore, education is fundamental to any clinical ultrasound program. A SWCUS would be involved in organizing teaching sessions for the various hospital departments. For example, initiation of quarterly ultrasound ground round lectures held by different departments. It would also help to organize training seminars for providers to help decrease repetitive efforts amongst the different departments.
Credentialing
Credentialing is essential to ensuring accountability and competency amongst providers in a department, hospital or organization. A centralized quality assurance program would increase the ability for institution-wide research on outcomes, comparative effectiveness and increase in patient satisfaction. A SWCUS would be responsible for creating a general credentialing process for the hospital system. This may include a decision on a standardized image archiving program and selecting of basic ultrasound studies to be credentialed. The SWCUS may also help with certifying leaders in different departments to perform QA for their members and to develop department specific credentialing guidelines.
Workflow Solutions
One significant restriction to hospital wide use of clinical ultrasound is if the imaging studies are unable to be associated with patient medical records or if different departments use different image archiving programs. This impairs communication amongst providers and could have clinical implications in the future. The SWCUS would coordinate with different hospital departments and the IT department to establish a system wide image archiving system and generate workflow solutions to have studies interact with the electronic medical record system.
Equipment Purchases and Maintenance
Whether it be for bulk sales of ultrasound gel or complete ultrasound machine purchases, the SWCUS can help your hospital to negotiate with manufacturers. Internally, the SWCUS would be able to work with the biomedical engineers to create sanitization and maintenance guidelines to ensure equipment reaches their proper life expectancy.
Reimbursement and Billing
In the United States, there are billing codes for ultrasound services for professionals. In some hospital systems, CUS is not routinely reimbursed. Since cooperation between physicians and coders is essential for billing optimization within your system, the SWCUS can work with coders to establish a reimbursement system for credentialed studies. This can lead to financial support for initial and future equipment, infrastructure, standardized IT workflow development, as well as ongoing maintenance.
In summary, a SWCUS can help to improve clinical ultrasound at a system wide level. As an emergency physician, you are prepared to become a champion for clinical ultrasound not only in your department, but for your entire hospital system. Start the conversation about SWCUS with your administration by bringing supporting literature. There are multiple articles and lectures that can be found on the web to educate others about a SWCUS.
Ultrasound Saves
Cardiac Tamponade Secondary to Pacemaker Perforation of the Myocardium: A Case Report
John Wesley, MS4; Matthew Flannigan, DO; and Sara Urquhart, MS3
Introduction
The use of pacemakers has been steadily increasing over the past decade as 3 million people worldwide currently have a pacemaker, with over 600,000 new ones being implanted every year.1 Various complications from pacemaker lead placement can arise with pneumothorax being the most common (1.9%-3.7%), followed by lead displacement (0.5%-4.8%) and myocardial perforation (0.37%-1.0%).2 While myocardial perforation is rare, it can cause significant pathological sequelae including cardiac tamponade and hemothorax. This case report involves an 88-year-old male who presented with cardiac tamponade secondary to myocardial perforation by a pacemaker lead which was placed nine days prior to onset of symptoms.
Case Report
An 88-year-old male presented to the emergency department (ED) with two hours of chest pain radiating to the neck bilaterally. The pain worsened with supine position.. He reported placement of a right ventricular permanent pacemaker replaced nine days prior to onset of symptoms. The pacemaker was placed for symptomatic AV node dysfunction. The patient had taken aspirin prior to presentation.
Vital signs were notable for a blood pressure of 70/50 and heart rate of 60. Other vital signs were within normal limits. The patient appeared pale with dry mucous membranes. Lungs were clear to auscultation, and cardiac exam showed a regular rate and rhythm with normal S1/S2 heart sounds.
Initial laboratory results showed a white blood cell count of 11,130/mL, hemoglobin of 9.20 g/dL, and Troponin T blood level of 0.045 ng/mL. The remainder of the lab results were within normal limits.
EKG showed an AV paced rhythm at 61 bpm with no acute ischemia. A point-of-care echocardiogram (ECHO) was performed. The ECHO revealed a moderate circumferential pericardial effusion with pacemaker perforation into the myocardium and right atrial free wall diastolic collapse consistent with tamponade physiology. This was subsequently confirmed by CT of the abdomen and thorax.
The patient was placed on Levophed for persistent hypotension and was sent to the cardiac cath lab for an emergent pericardiocentesis. 325 mL of bright red blood was removed and a pericardial drain was placed. The perforating pacemaker lead was removed, and a new lead was placed under ECHO guidance. The next day the patient reported improvement in symptoms and was hemodynamically stable with his blood pressure returning to a baseline of 142/66 mm/Hg. Repeat chest CT confirmed proper lead placement and complete resolution of the pericardial effusion. The pericardial drain was removed and the patient was discharged home in stable condition two days after admission.
Discussion
Myocardial perforation is a relatively rare complication of pacemaker lead placement. According to the National Cardiovascular Data Registry 444,251 pacemakers were placed between 2006 and 2011 with only 625 (0.14%) of them resulting in myocardial perforation.3 If myocardial perforation occurs, it is associated with significantly greater risks of morbidity (OR 27.5, 95% CI) and mortality (OR 17.7, 95% CI).3
Perforation typically occurs during placement of the device, but delayed perforation can occur several days or weeks after the procedure as well.4 Most cases of pacemaker lead perforation occur acutely, or within 24 hours of placement.5 Subacute perforations, which occur between 24 hours and one month, are less common than acute perforations at a rate of 24% to 76% respectively.6
Various factors including previous myocardial infarction, dilated heart chambers, and increased tension on the lead increase the risk of perforation.5 The most common site for perforation is through the right atrial wall as it is on average 2-3 mm thick in comparison to the right ventricle which ranges from 4-5 mm.5 However, most reported cases show increased mortality rates amongst patients with a right ventricular perforation as opposed to a right atrial perforation.5
It is important to ensure a timely diagnosis of pacemaker perforation as a delay in intervention can lead to worse morbidity and mortality. Point-of-care echocardiography has become an invaluable tool for emergency medicine and intensive care physicians to evaluate their differential diagnosis for patients presenting with chest pain and hypotension (Figure 3). Perforation is typically diagnosed by chest X-ray, ECHO, or CT of the thorax. It is important to note that if the X-ray and ECHO are initially negative, but perforation is still suspected, a CT of the thorax should still be performed as it is considered the gold standard.8
Conclusion
With the increasing use of pacemakers, it is important to consider myocardial perforation in a patient who presents with chest pain and hypotension in the setting of a recent pacemaker placement. Early detection of these symptoms and use of diagnostic tests such as chest X-ray, ECHO and CT can significantly improve outcomes in these patients.
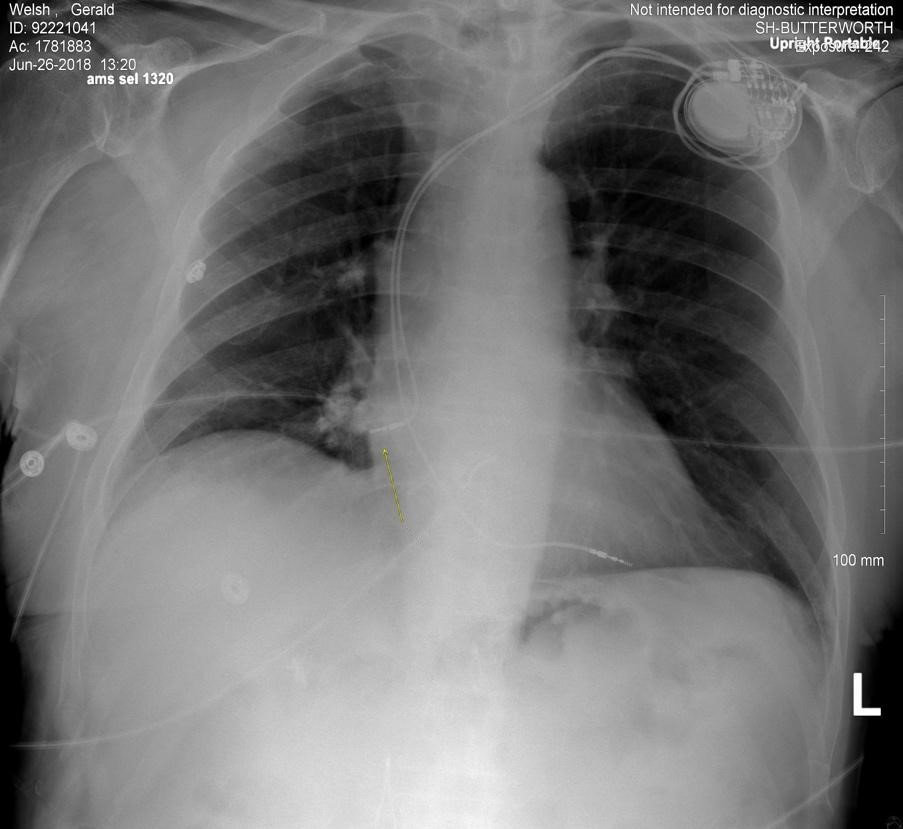
Figure 1. Chest X-ray showing perforation of cardiac pacemaker lead through the right atrium
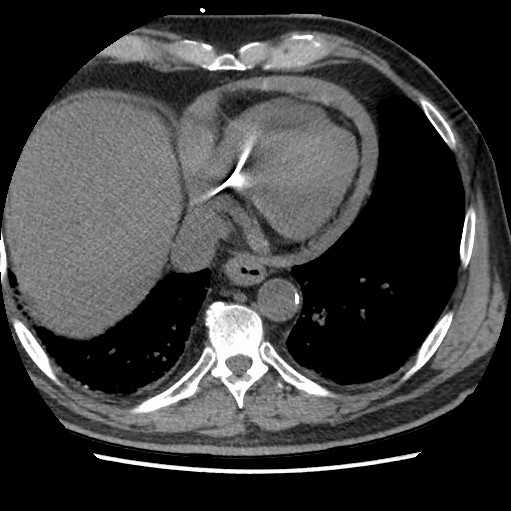
Figure 2. CT of the thorax showing a moderate circumferential pericardial effusion
|
Chest pain with hypotension |
Echo Findings |
|
Cardiac Tamponade |
|
|
Acute Decompensated Systolic Heart Failure |
|
|
Acute Decompensated Diastolic Heart Failure |
|
|
ST-Elevation Myocardial Infarction (STEMI) |
|
|
Aortic Dissection |
|
|
Pulmonary Embolism |
|
Table 1. Focused cardiac ultrasound findings for chest pain with hypotension
References
- Wood, Mark A., and Kenneth A. Ellenbogen. “Cardiac Pacemakers From the Patient’s Perspective.” Circulation, vol. 105, no. 18, 2002, pp. 2136–2138., doi:10.1161/01.cir.0000016183.07898.90.
- Vanezis, Andrew Peter, et al. “Pacemaker Leads and Cardiac Perforation.” JRSM Open, vol. 8, no. 3, 2017, p. 205427041668143., doi:10.1177/2054270416681432.
- Carlson, Mark D., et al. “Lead Perforation: Incidence in Registries.” Pacing and Clinical Electrophysiology, vol. 31, no. 1, 2007, pp. 13–15., doi:10.1111/j.1540-8159.2007.00943.x.
- Banaszewski, Marek, and Janina StÄ™piÅ„ska. “Editorial Right Heart Perforation by Pacemaker Leads.” Archives of Medical Science, vol. 1, 2012, pp. 11–13., doi:10.5114/aoms.2012.27273.
- Cano, Óscar, et al. “Incidence and Predictors of Clinically Relevant Cardiac Perforation Associated with Systematic Implantation of Active-Fixation Pacing and Defibrillation Leads: a Single-Centre Experience with over 3800 Implanted Leads.” Europace, 2016, doi:10.1093/europace/euv410.
- Khan, Mohammed N., et al. “Delayed Lead Perforation: A Disturbing Trend.” Pacing and Clinical Electrophysiology, vol. 28, no. 3, 2005, pp. 251–253., doi:10.1111/j.1540-8159.2005.40003.x.
- Piekarz, Justyna, et al. “Heart Perforation in Patients with Permanent Cardiac Pacing – Pilot Personal Observations.” Archives of Medical Science, vol. 1, 2012, pp. 70–74., doi:10.5114/aoms.2012.27284.
Ultrasound-guided Pericardiocentesis
Roberta Pritchard, MD; Michelle Clinton, MD; and Jonathan Nogueira, DO
Case
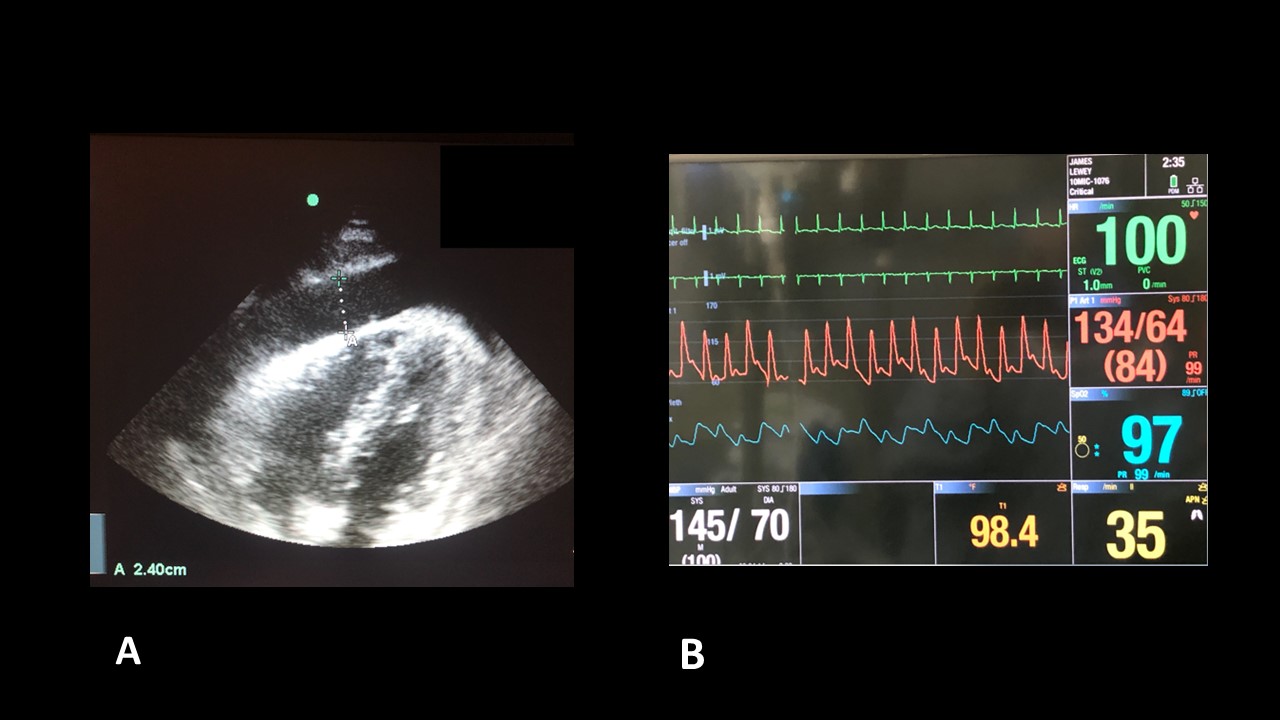
72-year-old male with past medical history of hypertension, hyperlipidemia and previous tobacco use who presents to the emergency department with shortness of breath and chest pain. The pain is a dull, pressure sensation worse when he lays flat. He describes a choking sensation with swallowing breakfast this morning and dyspnea on exertion. He has progressively worsened this week. Vital signs are notable for tachycardia at 104 and a blood pressure of 90/42 saturating 94% on 8L nasal cannula. Bedside ultrasound demonstrated a large pericardial effusion (Image 1a). The patient’s monitor was also noticed to show pulsus paradoxus that was maintained even after the pericardiocentesis (noting improved blood pressure) and further resuscitation (Image 1b).
Discussion
Pericardial effusions are most commonly caused by pericarditis. Other common causes include malignancy, trauma, uremia, and radiation therapy. Pericardial tamponade is a complication of pericardial effusions and manifests on ultrasound as chamber collapse (Video 1, Video 2, and Video 3) and variation in the filling of the right and left ventricle in relation to the respiratory cycle. As demonstrated in Image B the heart may demonstrate electrical alternans, this phenomenon visualized on electrical tracing is secondary to the physical swinging motion of the heart (Video 4). A pericardial effusion can appear similar to a pleural effusion.1 However, the pericardial effusion should only extend anterior to the descending aorta.
Ultrasound guided pericardiocentesis drastically reduces complication rates from an estimated 15% to 4.7% and is within the scope of an emergency physician. There are two approaches, the parasternal/apical approach and the subxiphoid approach.1,3 (Note: This procedure should be done in consultation with Cardiology unless the patient is actively decompensating).
Video 1: Right Ventricular Wall Collapse
Video 2: Right Ventricular Wall Collapse 2
Video 3: Right Atrial Wall Collapse
Video 4: Apex swinging in pericardial effusion
Ultrasound guided pericardiocentesis drastically reduces complication rates from an estimated 15% to 4.7% and is within the scope of an emergency physician. There are two approaches, the parasternal/apical approach and the subxiphoid approach.1,3 (Note: This procedure should be done in consultation with Cardiology unless the patient is actively decompensating).
Procedure
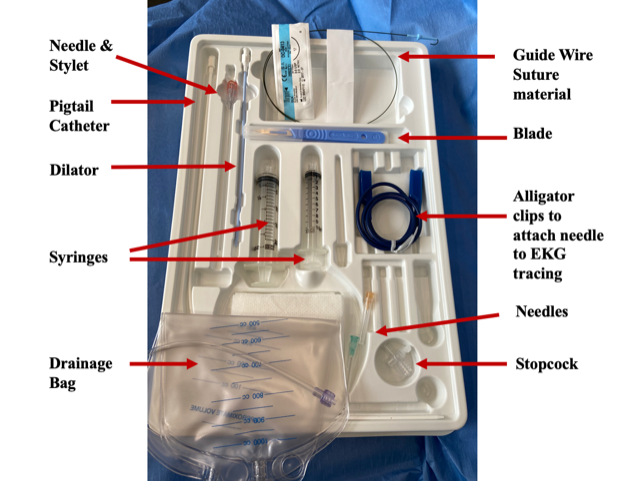
Supplies Set-Up (Image 2)
- Create a sterile field, including ultrasound probe with sterile cover and sterile gel.
- Place a phased-array or linear probe in the standard cardiac windows to identify the place where there is the largest fluid collection closest to the needle pathway.
- An 18 gauge or larger needle on a 10 mL syringe facilitates control of the needle. Larger bore needles allow for easier drainage and facilitate insertion of a pigtail via Seldinger technique.
Technique
- Two techniques for ultrasound guided pericardiocentesis are are described below:
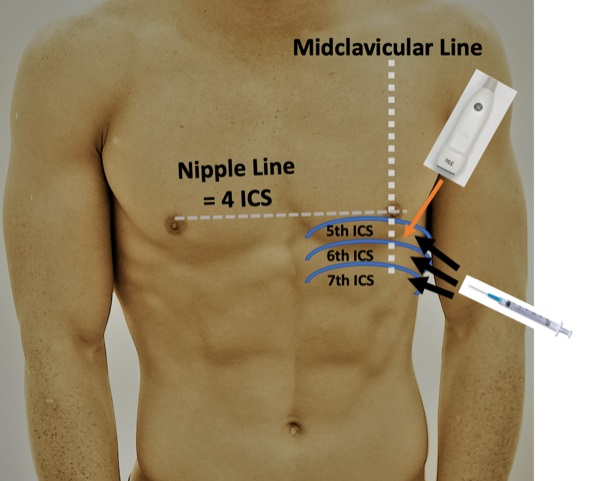
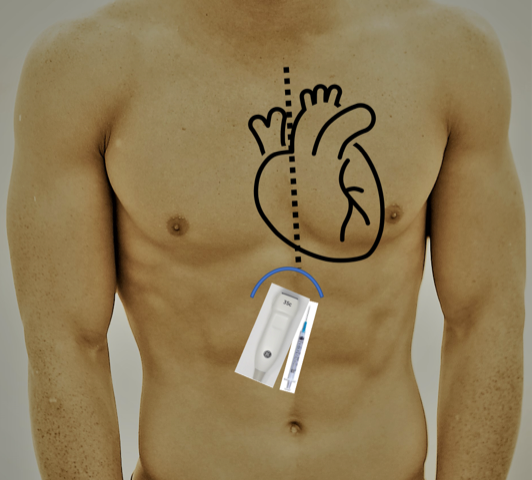
- Parasternal/apical Approach (Image 3/4):
- For the parasternal/apical approach, position the patient in the left lateral decubitus if possible.
- The largest fluid collection should be between the standard parasternal and apical view over the fifth, sixth, or seventh intercostal space between the midclavicular and midaxillary lines. Direct the needle in plane with the long axis of the probe visualizing the needle track avoiding any superficial vessels. The needle should be directed towards the right shoulder and advanced over the rib to avoid theneurovascular bundle. Track the needle along the long axis and visualize it entering the pericardial effusion.1,2 (Video 5).
- Parasternal/apical Approach (Image 3/4):
Video 5: Apical Pericardiocentesis with needle visualized
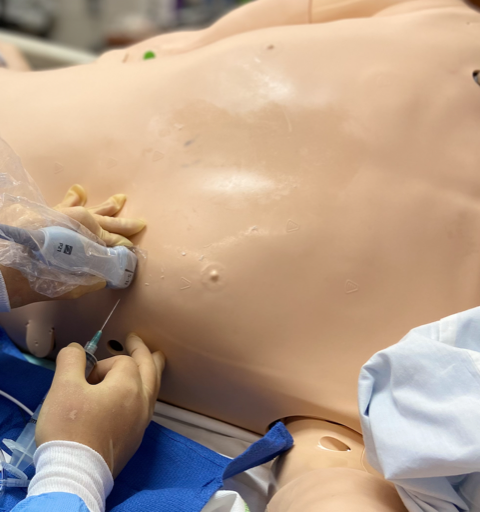
- Subxiphoid Approach (Image 5/6):
- For the subxiphoid approach, the patient is in the supine position.
- The most optimal view of the subxiphoid four-chamber view should be obtained, and the pathway of the needle visualized. Ideally, this would be from the subxiphoid angle, however this may be complicated by space limitations. It may also be possible to visualize the needle entering through the pericardium via the apical four-chamber view with the needle advancing from the subxiphoid approach. If choosing this method visualize the planned pathway of the needle to ensure there are no in-line structures including the liver prior to insertion of the needle.1,3
- The needle is inserted 1 cm inferior to the left of the xiphoid process angled at 30 degrees to the skin aiming for the left shoulder. Aspirate during insertion, stop once fluid is returned.4 (Video 6)
Video 6: Subxiphoid with needle
- Aspirate fluid. If the fluid is bloody and placement location is a concern, 3-5 mL of the aspirated fluid can be re-injected and can often be visualized re-entering the pericardial space confirming the location. Once fluid is freely aspirated, a pigtail catheter can be placed using standard Seldinger technique. Note the clip below demonstrating the improvement in the pericardial effusion and hemodynamics after a successful pericardiocentesis using the subxiphoid approach (Video 7).
Video 7: Subxiphoid with improved hemodynamics
Images
Image 1A & 1B.
Image 2.
Image 3.
Image 4.
Image 5.
Image 6.
References
- Otto, C. Textbook of Clinical Echocardiography (6th ed.). Chapter 10: Pericardial Disease. Pp 268-287. Philadelphia, PA: Elsevier; 2018.
- American College of Emergency Physicians: Emergency ultrasound guidelines-2008. Ann Emerg Med 53:550, 2009.
- Dewitz A, Jones RA, Goldstein JG, Stone MB. Chapter 22. Additional Ultrasound-Guided Procedures. In: Ma O, Mateer JR, Reardon RF, Joing SA. eds. Ma and Mateer's Emergency Ultrasound, 3e. McGraw-Hill
- Roberts J, Hedges J, editors. Clinical Procedures in Emergency Medicine. 7th ed. Chapter 16: Pericardiocentesis. pp 309-331. Philadelphia, PA: Elsevier, Inc; 2018.
Letter to the Editors
What stood out to you from this issue of the POCUS Report? Have a question, idea, or opinion? Alexis Salerno, MD FAAEM and Melissa Myers, MD FAAEM welcome your comments and suggestions. Submit a letter to the editor and continue the conversation at https://form.jotform.com/90834609522156.
Get Involved
We encourage original submissions to this e-newsletter and to our recurring section in Common Sense. Please contact us with any questions at EUS@aaem.org. Stay Calm and Ultrasound On!