Fall 2019
Welcome from EUS-AAEM
The Emergency Ultrasound Section of the American Academy of Emergency Medicine (EUS-AAEM) is founded to foster the professional development of its members and to educate them regarding point of care ultrasound. This group will serve as a venue for collaboration among medical students, residents, and practitioners who are interested in point of care ultrasound. The purpose of our group is to augment the knowledge and expertise of all emergency medicine specialists and to advocate for patient safety and quality care by endorsing bedside ultrasound. Membership is not limited to fellowship trained physicians. All emergency medicine practitioners passionate about ultrasound are welcome to join and participate.
We are proud to publish our e-newsletter with original contributions from many of our members. We encourage all members to submit for future additions. Topics include but are not limited to educational, community focus, interesting cases, resident and student section, and adventures abroad.
For more information visit us online at: www.aaem.org/EUS.
In this Issue:
-
President's Message
-
Ultrasound Building Blocks: More than Meets the Eye: Using Ultrasound to Assess for Retinal Detachment in the Emergency Department
-
Resident Section: Obtaining Cardiac Output using Bedside Echocardiography
-
Ultrasound Highlights
-
Fellow Section: Development of a Focused Designation of Clinical Practice in Ultrasound
-
US Guided Procedures: Ultrasound-guided Glenohumeral Intra-articular Hematoma Block for Anterior Shoulder Dislocation
-
Prehospital POCUS: Lung Ultrasound is the Most Valuable Prehospital POCUS Application
-
Administrative Corner: The Q’s & A’s of Ultrasound Quality Assurance
-
Ultrasound Saves: Point of Care Ultrasound for Assessment of Soft Tissues
-
From Journal to Practice: Cardiac Standstill: How Do You Know When It’s Time to Stop?
-
Letter to the Editors
-
Get Involved
President's Message
Hello all current and new Emergency Ultrasound Section (EUS-AAEM) members! We are over 500 members strong and growing.
These are exciting times for our section and emergency ultrasound in general with an alphabet soup of activity. Nationally, there is the upcoming emergency ultrasound Fellowship match day through the National Residency Match Program (NRMP). The American Board of Emergency Medicine (ABEM) through the American Board of Medical Specialties (ABMS) is moving forward with a new form of subspecialty for emergency ultrasound, called the Focus Practice Designation (FPD) in Advanced Emergency Medicine Ultrasound (AEMUS). Finally, the Emergency Ultrasound Fellowship Accreditation Council (EUFAC) is preparing to certify the first emergency ultrasound programs.
As a section, we are working hard to help you and support our community with several initiatives that are at different stages of development. Currently we are working on the following:
- Skills Verification Program (SVP) for AAEM20 in Phoenix, AZ: In conjunction with the Emergency Ultrasound – Beginners pre-conference course, we will be rolling out a process for Emergency Physicians (EPs) who are no longer in training to have proctored emergency ultrasound exams by emergency ultrasound experts to facilitate additional emergency ultrasound privileges at local facilities.
- Regional Emergency Ultrasound Courses: We are scheduled to have our first regional emergency ultrasound course in New York in February 2020 with the goal of increasing access to affordable emergency ultrasound workshops outside the annual scientific assembly.
- POCUS Report: This is our primary way to communicate to you, our members, and share relevant and exciting content regarding emergency ultrasound and our section.
- Future Initiatives: Some of these include The Definitive EUS Resource website and medical student/resident/fellowship emergency ultrasound opportunities.
If you have any questions, please feel free to contact me at business@thechinfamily.com.
Sincerely,
Eric Chin, MD MBA FAAEM
EUS-AAEM President 2019-2020
Ultrasound Building Blocks
More than Meets the Eye: Using Ultrasound to Assess for Retinal Detachment in the Emergency Department
Kristyn J. Smith, DO; Victoria Kneller, DO; Molly Furin, MD; and Allison Zanaboni, MD FAAEM
Case
A 55-year-old male with a past medical history of hypertension and type II diabetes presented to the emergency department with atraumatic decreased vision and floaters of the left eye which had been worsening for three days. He denied fevers, headaches, nausea, vomiting, and eye pain. The patient did not wear contacts or corrective lenses.
On exam vital signs were within normal limits. The pupils were 2mm and briskly reactive bilaterally. There was no chemosis, scleral injection, or purulent drainage bilaterally. The head and eyes were atraumatic and without periorbital skin changes suggestive of trauma or infection. Extraocular movements were intact bilaterally. Fundoscopic exam was attempted but equivocal given a lit room and lack of pupillary dilation. There was significant vision deficit in the left eye. The patient was unable to see any letters on the Snellen chart and was only able to see light, not movement, with the affected eye.
Based on the patient’s exam, what diagnoses are you considering? What would be your next steps?
Diagnosis
Given a non-diagnostic fundoscopic exam with appreciable vision deficit, our next step was to evaluate the patient using Point of Care Ultrasound (POCUS).
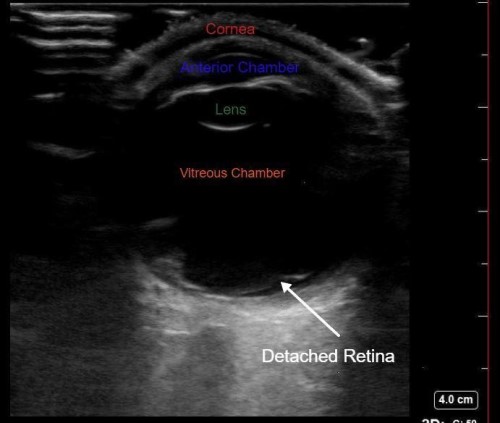
POCUS of the left eye revealed a hyperechoic membrane floating in the vitreous chamber, consistent with a diagnosis of retinal detachment. Ophthalmology was emergently consulted and an ophthalmologist performed Brightness scan, diagnostic two-dimensional ocular ultrasound confirmed the diagnosis. The patient was referred to a retinal specialist and underwent outpatient surgery.
Discussion
Eye complaints ranging from vision loss to pain comprise approximately 2-3% of emergency department visits annually.1 Delayed diagnosis of many emergent ocular diseases can result in permanent vision loss causing life altering disability and morbidity. Therefore, it is important for emergency physicians to diagnose diseases of the eye quickly and competently. Point of care ultrasound (POCUS) is an effective method of diagnosing ocular pathology at the bedside.
Ocular Ultrasound Technique2,3
- Position the patient: Place the head of the bed either supine or at a 45-90 degree angle
- Prepare the site:
- Instruct the patient to close the affected eye and place a large Tegaderm over the eye to be examined. A very thin layer of Vaseline or gel can be applied to the lash line prior to Tegaderm placement to prevent eyelash removal when removing the Tegaderm.
- Place a copious amount of ultrasound gel onto the Tegaderm over the affected eye and begin scanning.
- Start scanning:
- Using the linear transducer, scan through the eye in both the sagittal (probe indicator pointing cephalad) and transverse (probe indicator to the patient’s right) planes. Both static (patient looks straight ahead and keeps the eye stationary) and kinetic (patient moves the eyes in an up, down, left, right motion) views should be obtained.
- To ensure you do not apply too much pressure to the orbit, gently rest your pinky finger on the bridge of the patient’s nose or side of the face.
Things to look for:
- All structures of the eye: from anterior to posterior: cornea, anterior chamber, lens, vitreous /posterior chamber, retina, optic nerve.
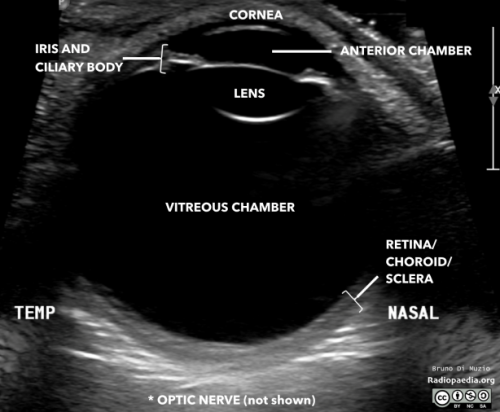
- Echogenic material: May be foreign bodies, blood, or retinal/vitreous detachment. You should make note of whether the hyperechoic material moves with patient eye movement and whether the material is attached to the optic nerve or not.
- Increased Intracranial Pressure (ICP): Measuring the ICP is most useful when assessing patients with headache or when increased ICP is suspected. 3mm posterior to the globe measure the diameter of the optic nerve sheath. Obtain the optic sheath diameter for both eyes and average the values. Increased ICP is suspected if the diameter is greater than 5mm. A good memory tool to use is “3 down, 5 across”.
Retinal Detachment vs. Posterior Vitreous Detachment
Retinal detachment (RD) is separation of the sensory retina from the underlying retinal pigment epithelium which leads to loss of retinal blood supply.1 Symptoms of retinal detachment include floaters, flashes, unilateral central or peripheral vision loss, or “curtain closing” vision. Retinal detachment is an emergent condition that requires ophthalmology consult within 24 hours and surgical repair by a retinal specialist.1
Posterior vitreous detachment (PVD) occurs as the vitreous humor degenerates and separates from the retina.1 PVD is caused by advanced age. Vitreous detachment alone typically presents with visual floaters, but is not vision threatening and most patients experience progressive improvement in symptoms; however, vitreous detachment can lead to future retinal detachment warranting close ophthalmology follow up.
It can be difficult to distinguish between retinal detachment, a true ocular emergency, and vitreous detachment based on symptoms alone. Both patients with retinal detachment and vitreous detachment may have floaters and flashes. To distinguish between these conditions, it is important to remember the anatomy of the eye and that the retina is anchored at two sites: posteriorly at the optic nerve and anteriorly at the ora serrata. In retinal detachment, POCUS will reveal a hyperechoic membrane within the vitreous body that moves with eye motion, however the membrane will remain tethered at two spots.4 With the proper amount of gain you can often see an echogenic line connecting the optic nerve and the retina, further indicating the presence of a RD. In PVD, POCUS will show detached hyperechoic material in the posterior chamber that will cross the optic nerve.4
Literature shows ocular POCUS performed by ED providers is both sensitive and specific for RD, PVD and other ocular pathology. One of the first studies examining the sensitivity and specificity of ocular ultrasound performed by ED physicians and residents showed a 100% sensitivity and 97.2% specificity overall.5 This 2002 study found concordance between diagnoses based on ED ocular POCUS and ophthalmologist performed exams in 60 cases out of 61.5 An article in the April 2019 edition of JAMA from a prospective cohort study showed a sensitivity of 96.9% and specificity of 88.1% for the diagnosis of retinal detachment.6 Results from another recent prospective cohort study demonstrated ocular ultrasound had a specificity of 98.7% and sensitivity of 97.8% when compared to an ophthalmologist-performed bedside ocular exam.7 A metanalysis that included 11 observational studies showed the sensitivity of POCUS in diagnosing RD to be 92% (CI 67.2% - 98.5%) and specificity of 91.4% (84.9 – 95.3%).8 Overall ocular ultrasound is sensitive and specific for the diagnosis of retinal detachment when used by trained emergency medicine providers.
References
- Walker RA, Adhikari S. Eye emergencies. In: Tintinalli JE, Stapczynski S, Ma OJ, Yealy DM, Meckler JD, Cline DM, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. Chicago, IL: McGraw-Hill Medical; 2011:236:1517-1549.
- Bargren, Anna. EM in 5: Ocular Ultrasound.
- Meer, J; Taylor, T; Beck, S. Emergency Ultrasound: Bedside Ultrasound for Ocular Emergences. Emergency Medicine. 2014. October; 46(10): 466-668.
- Adhikari, Srkar. Small Parts – Ocular Ultrasound. Sonoguide: Ultrasound Guide For Emergency Physicians. 2008. <https://www.acep.org/sonoguide/smparts_ocular.html>.
- Blaivas, M, Theodoro, D, Sierzenski, PR. A study of bedside ocular ultrasonography in the emergency department. Acad Emerg Med. 2002. Aug; 9(8): 791-799.
- Lahham S, Shniter I, Thompson M, et al. Point-of-Care Ultrasonography in the Diagnosis of Retinal Detachment, Vitreous Hemorrhage, and Vitreous Detachment in the Emergency Department. JAMA Netw Open. Published online April 12, 20192(4):e192162.
- Ojaghihaghighi, S, et al. Diagnosis of Traumatic Eye Injuries With Point-of-Care- Ocular Ultrasonography in the Emergency Department. Annals of Emergency Medicine. 2019. Sept; 74(3): 365-371.
- Gottileb, M, Holladay, D, Peksa GD. Point of Care Ocular Ultrasound for the Diagnosis of Retinal Detachment: A Systematic Review and Meta-Analysis. Acad Emerg Med. 2019 Aug; 26(8):931-939.
Resident Section
Obtaining Cardiac Output using Bedside Echocardiography
Daniel Boron-Brenner, DO
HPI: A 29 year old female with a past medical history of heart failure with reduced ejection fraction (last echo 50% in January 2019), ESRD on hemodialysis, hypertension, and a 12-pack year smoking history presented to the emergency department with a chief complaint of progressive shortness of breath and associated “room spinning” for one day. Her last hemodialysis session to completion was the day of presentation. The patient reports not missing prior sessions as well as compliance with her medications. She actively follows with a cardiologist. Point of care ultrasound was used in the evaluation of ejection fraction and cardiac output was obtained using left ventricular outflow tract diameter (LVOT) area and velocity time integral (VTI).
Echo:
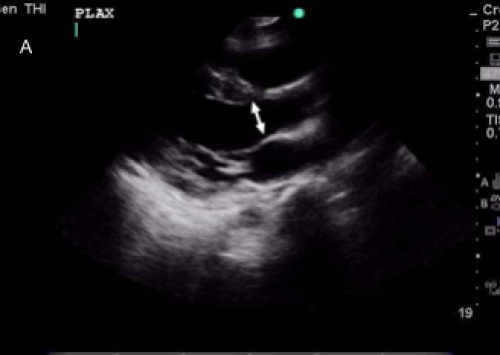
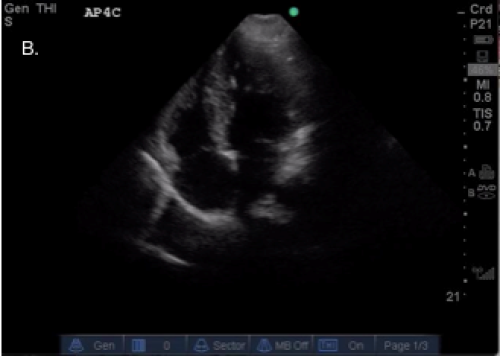
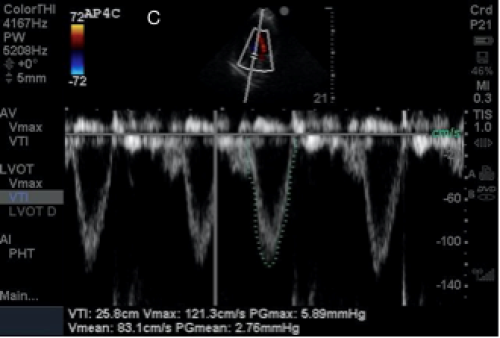
A: A para-sternal long axis view. Note, doublearrows indicate the LVOT
B: An apical 4-chamber view
C: A screen depicting the VTI on a Sonosite Mturbo ultrasound machine
Discussion:
Transthoracic echocardiography can be used to assess stroke volume, and more importantly cardiac output, in patients for whom there is concern for depressed function.
Since the technique was first discussed in the 1980s, cardiac output obtained via bedside ultrasonography has been found to correlate highly. In 2012, the Dinh group published a study in the American Journal of Emergency Medicine comparing measurements of cardiac index (including LVOT area and VTI) obtained by ED physicians and a trained sonographer. The results of this study showed an average correlation coefficient of 0.82, demonstrating that emergency physicians can accurately perform this measurment.1 Additionally, in 2018 the Betcher group performed a prospective, observational study consisting of 53 patients in a single-site academic emergency medicine training program. They found that the average time to obtain information about cardiac output for their ultrasound team, consisting of an ultrasound fellow and an ED resident, was just three minutes and eight seconds. 2
Calculating cardiac output can be achieved using basic ultrasound equipment (including older-generation ultrasound machines) and an understanding of some simple mathematical and physiologic concepts. First, we need to understand a few background concepts and formulas:
- Cardiac output (CO) = Stroke volume (SV) x heart rate (HR). A normal cardiac output is 4-6 L/min.
- Using the pulsed waveform Doppler on ultrasound, we can assess stroke volume by calculating the product of the left ventricular outflow tract (LVOT) area and the range of velocities of the blood flowing across the LVOT, known as the velocity time integral (VTI).
- The area of the LVOT is measured assuming that the outflow tract is shaped like a cylinder, whose cross-sectional area is a circle, the diameter of which can be assessed using echocardiography in the parasternal long axis view.
- Therefore, SV (cm3)=π(LVOTd/2)2 x VTI (cm)
Measuring LVOT
To measure the LVOT area, obtain a parasternal long axis view and measure the LVOT at midsystole, or the point of maximal separation of the mitral valve leaflets. Measure from inner edge to inner edge at the level of the aortic annulus and don’t forget to measure perpendicular to the direction of the LVOT. Once you have obtained this measurement, which is equivalent to the diameter of the outflow tract, input it into the formula above.
Measuring VTI
In order to obtain the VTI, you will first need to obtain an apical 5-chamber view on echo. You will then use the pulsed wave Doppler function to measure velocity of flow across the LVOT. The pulsed wave Doppler gate (the line with two central hash marks running perpendicularly through the middle of it) should be placed over the LVOT, in the direction of flow out of the LVOT. This will produce a waveform tracing of the velocities of blood flowing out of the LVOT with each heartbeat. Next using the ultrasound cardiac calculator function, select LVOT VTI (usually found under “CO” or “Ao” in the cardiac measurement headings) and outline one of the waveform tracings. The resulting value will be your VTI.
Calculating SV
Once you have obtained the LVOT area and the VTI, it’s a simple matter of inputting the values into a formula to obtain the stroke volume. This can then be multiplied by the heart rate to give you the patient’s cardiac output. Alternatively, some machines will have the ability to complete the entire calculation for you, making the process even simpler.
Limitations
Limitations do exist for obtaining cardiac output utilizing LVOT area and VTI. These include errors caused by ineffective operator technique, device limitations and patient factors including compliance with exam, body habitus, and acuity of presentation.
From an operative perspective, the study mentioned earlier by the Betcher group found that times to accurate assessment decreased with advancements in training level, indicating that junior trainees (or those with a concomitant dearth of experience in ultrasound) potentially required longer times to appropriate assessment.3 Additionally, the initial assumption that the LVOT area is always cylindrically shaped doesn’t necessarily take anatomic variation or variation during systole into consideration. Consequently, multiple measurements of the LVOT during systole can have similar, but not identical, values.
There is also a risk of inaccurate assessment with hemodynamic conditions affecting cardiac function, including atrial fibrillation. It is recommended that these patients require averages of multiple assessments, sometimes between five and ten cycles of the pulse wave, to render a precise VTI.
Conclusion
In this case our patient had a LVOT diameter of 1.8 cm and a VTI of 25.8. This calculates to a stroke volume of 66mL which is in the normal range. Although limitations should be taken into consideration, utilization of ultrasound-derived stroke volume and subsequently cardiac output provides another tool for the echocardiographic toolbox. Using stroke volume with other sonographic studies of hemodynamic status and function, including IVC evaluation and assessment of E-point septal separation (EPSS), can provide a potentially powerful window into the assessment and care of the critically ill patient.
References
- Blankenship, J. and Mallin, M. (2019). Echocardiographic Assessment of Cardiac Output and Ejection Fraction. [online] Ucdenver.edu. Available at: http://www.ucdenver.edu/academics/ colleges/medicalschool/departments/EmergencyMedicine/Sections/ultrasound/education/ Documents/1%202%203.pdf [Accessed 2 Jun. 2019].
- Vermeulen, M. (2017, November 30). Management of Shock: Bedside Assessment of Cardiac Output. Retrieved from https://www.emra.org/emresident/article/management-of-shock-bedsideassessment-of-cardiac-output/
- Betcher, J., Majkrzak, A., Cranford, J., Kessler, R., Theyyunni, N., & Huang, R. (2018). Feasibility study of advanced focused cardiac measurements within the emergency department. Critical Ultrasound Journal,10(1). doi:10.1186/s13089-018-0093-4
- Dinh, V. A., Ko, H. S., Rao, R., Bansal, R. C., Smith, D. D., Kim, T. E., & Nguyen, H. B. (2012). Measuring cardiac index with a focused cardiac ultrasound examination in the ED. The American Journal of Emergency Medicine,30(9), 1845-1851. doi:10.1016/j.ajem.2012.03.025
Ultrasound Highlights
Finding the Target: Appendicitis on Ultrasound
Robinson, MD FAAEM
This lecture provides an introduction to performing limited abdomen ultrasound for appendicitis, including discussion of technique, identification of anatomical landmarks, recognition of appendicitis on ultrasound, and reviewing pearls and pitfalls to increase success rates.
Fellow Section
Development of a Focused Designation of Clinical Practice in Ultrasound
Melissa Myers, MD FAAEM and Alexis Salerno, MD
Emergency physicians have been an essential part of the development of Point-of-Care Ultrasound (POCUS). In the 1970s, POCUS started as part of the trauma resuscitation. Since then, emergency physicians have expanded the boundaries of POCUS to evaluate and treat a wide range of medical conditions. As early as 1988, emergency physicians began publishing on the use of bedside ultrasound in the emergency department. Within a few years, in 1991, both the American College of Emergency Physicians (ACEP) and the Society of Academic Emergency Medicine (SAEM) published policy statements regarding the utility of bedside ultrasound in the emergency department.1
Emergency physicians have also led the way in developing curriculum. The first published curriculum in 1994 by Mateer et al. has led to multiple well-developed curriculums based in educational research. Today, POCUS is considered an essential skill and was recognized as such in the 2013 Model of the Clinical Practice of Emergency Medicine. Modern emergency medicine residencies include rigorous and extensive training in POCUS with graduates performing a wide array of POCUS skills to diagnosis and treat their patients.
Some emergency physicians choose to pursue ultrasound training beyond that required during residency, by completing an emergency ultrasound fellowship. During one or two year fellowships, these physicians become experts in advanced ultrasound modalities and ultrasound education. The presence of ultrasound fellowship trained faculty at residency sites correlates with a higher number of faculty credentialed to perform ultrasound and may assist with quality assurance for ultrasound performed in the ED.2 Fellowship trained emergency physicians also continue to develop new ways to improve the use of POCUS and to study best practices for use on shift.
The Society for Clinical Ultrasound Fellowships (SCUF) currently lists 50 fellowships, though this list is not exhaustive, and does not include the military programs. Until recently, there has been no established way to recognize physicians who choose to pursue this extra training or to credential these fellowships. While some have chosen to pursue recognition through the exams offered by the American Registry for Diagnostic Medical Sonography or other similar organizations, these exams were not developed by emergency physicians and do not reflect the use of POCUS in the emergency department. As noted by Dr. Gibbons in the May/June 2019 edition of Common Sense, emergency physicians do not need these merit badges to legitimize our training.3
Following an extended debate and vote, members of these fellowships and ultrasound societies nationwide felt that attempting to establish a subspecialty board could have unintended consequences for the practice of POCUS by those who did not choose to pursue a fellowship. The alternative chosen was a “Focused Practice Designation (FPD).”
The FPD, which is approved by the American Board of Medical Specialties, “recognizes physicians who devote a substantial portion of their practice to a specific area of a specialty.”4 This will hope to recognize emergency physicians with expertise in emergency ultrasound beyond the requirements for ABEM certification. It will be a recognition developed by emergency physicians which will be specific to the requirements of our specialty. There will be three pathways to obtain this designation, the fellowship training pathway, the training-plus-practice, and the practice-only pathway.5
In the fellowship training pathway, physicians will complete an Advanced Emergency Medicine Ultrasound (AEMUS) fellowship accredited by the Emergency Ultrasound Fellowship Accreditation Council (EUFAC). The Society of Clinical Ultrasound Fellowships (SCUF) will be charged with the creation of this council. For those who do not know, the current SCUF website helps potential fellows compare various ultrasound fellowships and complete fellowship applications. In the future, the EUFAC will release regulations to obtain fellowship accreditation and a curriculum for the fellows. The curriculum will expand on the basic emergency medicine ultrasound knowledge by including advanced measurements and views. Although the curriculum has not been released yet, potential topics may include muscular tendon assessment, arterial doppler assessment or even cardiac diastology. The curriculum will most likely also cover administrative topics such as billing and workflow solutions.
In the training-plus-practice pathway, physicians must complete an acceptable non-EUFAC accredited fellowship. This pathway will most likely be for recent emergency ultrasound fellows who graduated prior to the date of the first accredited fellowship. The physician must also demonstrate 24 months of AEMUS practice including performing or supervising 300 studies per year and reviewing for quality assurance 500 studies per year. This pathway will only be available to physicians for five years from the date of the first EUFAC-accredited AEMUS fellowship. Those who are considering applying for this pathway, may wish to start logging ultrasound scans and QA’ed studies.
In the practice-only pathway, physicians must demonstrate 36 months of AEMUS practice with 300 performed or supervised studies and 500 reviewed studies. In addition, physicians will have to demonstrate additional knowledge in the area by prior work in leadership administration, publications, or teaching. This pathway will most likely be for more senior faculty that continue to have a strong interest in ultrasound. And just as in the training-plus-practice pathway, this will only be available to physicians for five years from the date of the first EUFAC-accredited AEMUS fellowship.
Physicians who meet the eligibility criteria will also need an appropriate verifier who can confirm the physician has the hand-eye-motor coordination to perform ultrasound tasks. Finally, physicians will be able to take a multiple-choice examination to gain FPD. The first exam is scheduled to be offered in 2022.
Through these pathways, emergency physicians who devoted significant time and attention to practicing point-of-care ultrasound will be able to obtain recognition of their expertise. This exciting development will likely continue to evolve over the next few years as ultrasound societies nationwide work together to develop the exam and fellowship credentialing guidelines. To keep updated on the progress of the AEMUS FPD check out the SCUF website at eusfellowships.com and don’t forget to check out EUS-AAEM newsletter, the POCUS Report.
References
- Kendall, J. L., Hoffenberg, S. R., & Smith, R. S. (2007). History of emergency and critical care ultrasound: the evolution of a new imaging paradigm. Critical care medicine, 35(5), S126-S130.
- Das, D., Kapoor, M., Brown, C., Ndubuisi, A., & Gupta, S. (2016). Current status of emergency department attending physician ultrasound credentialing and quality assurance in the United States. Critical ultrasound journal, 8(1), 6.
- Gibbons, Ryan. “Emergency Ultrasound Merit Badges...There’s No Need.” Common Sense, May/June 2019.
- Focused Practice Designation. Focused Practice Designation | American Board of Medical Specialties. https://www.abms.org/board-certification/focused-practice-designation/. Accessed August 18, 2019.
- Advanced EM Ultrasonography. American Board of Emergency Medicine. https://www.abem.org/public/become-certified/focused-practice-designation/advanced-em-ultrasonography. Accessed August 18, 2019.
US Guided Procedures
Ultrasound-guided Glenohumeral Intra-articular Hematoma Block for Anterior Shoulder Dislocation
Waroot Nimjareansuk, DO and Michael Rosselli, MD
Case Description
A 22-year-old male with a past medical history of seven prior right shoulder dislocations, presented with right shoulder pain, swelling, and deformity after taking off his shirt to shower. His vitals were within normal limits. His physical examination showed a right shoulder deformity with significantly limited range of motion. His distal sensation and pulses were intact with normal capillary refill. Point of care ultrasound of the right shoulder showed an anterior dislocation of the humeral head in relation to the glenoid. Subsequently, an ultrasound-guided glenohumeral intra-articular hematoma block with lidocaine was performed as described below.
An ultrasound guided intra-articular hematoma block with lidocaine is a safe and effective alternative to procedural sedation and should be considered first-line for the reduction of shoulder dislocations. A Cochrane review of five studies with 211 patients with acute anterior shoulder dislocations compared intra-articular lidocaine to intravenous analgesia with and without sedation. The review showed that there was no difference in successful closed manual reduction with fewer adverse events and less time in the emergency department with the intra-articular lidocaine group.1-2 In one randomized controlled trial (RCT) of 48 patients by Moharari et al., the mean length of stay was 140 minutes in the intra-articular lidocaine (IAL) group versus 216 minutes in the intravenous sedation (IVS) group with fewer complications in the intra-articular lidocaine group.3 Another RCT of 54 patients showed a mean length of stay of 103 minutes in the IAL group versus 154 minutes in the IVS group. The use of an ultrasound guided intra-articular hematoma block with lidocaine can shorten the patient’s emergency department stay with no difference in the success of the reduction.4
Procedure
- Create a sterile site at the lateral aspect of the affected shoulder. Depending on body habitus, place the low frequency (curvilinear) or high frequency (linear) probe at the posterior shoulder in transverse position. Slide the probe laterally until the glenoid and the head of the humerus come into view.
- Using an in-plane approach, inject 10-20cc of 1% lidocaine with a 20-gauge spinal needle into the glenohumeral joint space as shown in Figure 1. Be sure to aspirate blood prior to injecting lidocaine to ensure that you are in the intra-articular space.
- Wait approximately 10-15 minutes for the anesthetic to take effect, then reduce the affected shoulder and confirm reduction with point-of-care ultrasound as shown in Figure 2.
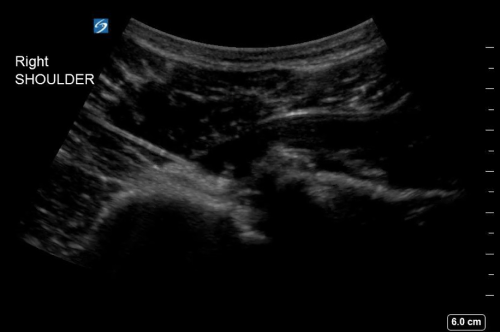
Figure 1. Ultrasound-guided glenohumeral hematoma block with lidocaine of a right anterior shoulder dislocation.
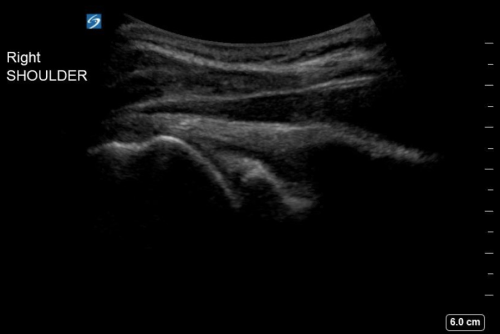
Figure 2. Post-reduction ultrasound of the right shoulder
References
- Wakai A, O’Sullivan R, McCabeA. Intra-articular lidocaine versus intravenous analgesia with or without sedation for manual reduction of acute anterior shoulder dislocation in adults. Cochrane Database Syst Rev. 2011;CD004919. PMID: 21491392.
- Hunger B, Wilbur L. Can Intra-articular Lidocaine Supplant the Need for Procedural Sedation for Reduction of Acute Anterior Shoulder Dislocation? Ann Emerg Med. 2012;59(6):513-14.
- Moharari RS, Kademhosseini P, Espandar R, Soleymani HA, Talebian MT, Khashayar P, Nejati A. Intra-articular lidocaine versus intravenous meperidine/diazepam in anterior shoulder dislocation: a randomized clinical trial. Emerg Med J. 2008;25(5):262-64.
- Orlinsky M, Shon S, Chiang C, Chan L, Carter P. Comparative study of intra-articular lidocaine and intravenous meperidine/diazepam for shoulder dislocations. J Emerg Med. 2002;22(3):241-45.
Prehospital POCUS
Lung Ultrasound is the Most Valuable Prehospital POCUS Application
Andrew Merelman, BS NRP FP-C
If you are going to perform one ultrasound exam in the prehospital setting, it should be the lung exam. POCUS Lung exam is an easy, quick exam that allows prehospital providers to rule-in or rule-out clinically significant pulmonary edema and can determine signs of pneumothorax. This means they can much more accurately determine the cause of dyspnea.
Lung Ultrasound to Distinguish Pulmonary Edema from COPD
You respond to a 70-year-old male patient with difficulty breathing. He appears in moderate to severe distress and worsening. He has a known history of congestive heart failure, emphysema, hypertension, and type II diabetes. Lung sounds are diminished bilaterally. Initial vital signs BP 188/98, HR 118, RR 30, SpO2 84% on RA. What are your next steps in assessment and treatment?
EMS providers are commonly faced with this scenario. An elderly patient with known history of both CHF and COPD in acute distress. It is often very challenging to differentiate between these two conditions. Auscultation is unreliable as a tool to determine specific etiology and is non-specific for diagnosis of pulmonary edema.1 The presence of hypertension is non-specific for CHF as many COPD patients experience it at baseline or during episodes of dyspnea. ETCO2 may be helpful if there are signs of bronchospasm, as indicated by a prolonged expiratory phase, but this finding may not always be present. Patients can also have a combination of bronchospasm and pulmonary edema. Despite these assessment difficulties, paramedics need to be able to treat the primary condition, each of which have very different priorities. Enter POCUS.
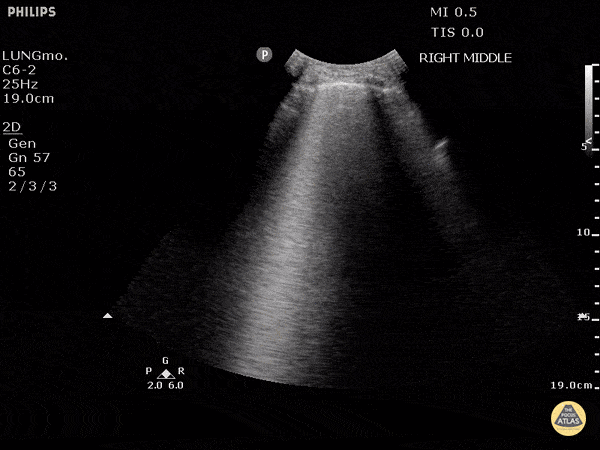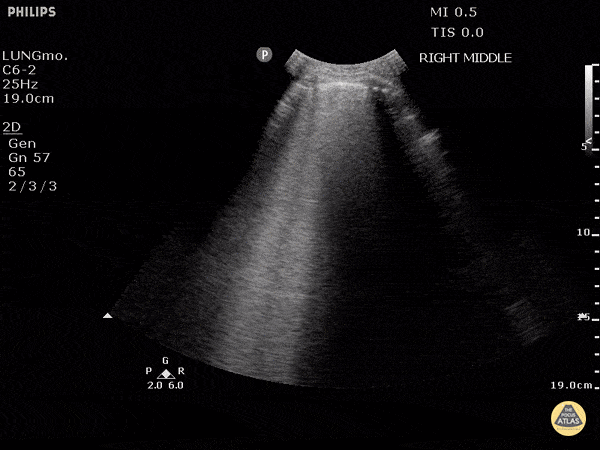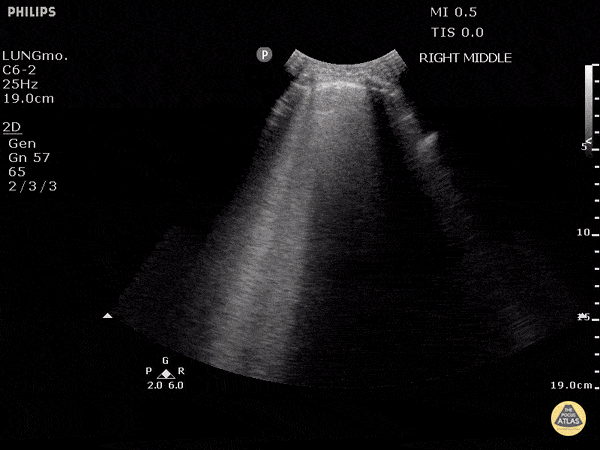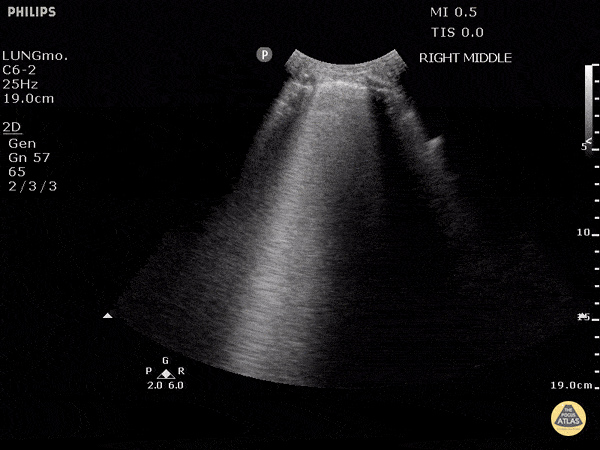
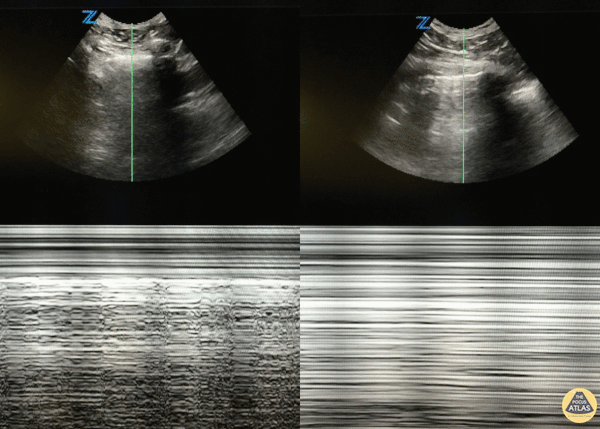
Studies have shown ultrasound is reliable for detecting pulmonary edema2-4 and can distinguish between COPD and CHF in the prehospital setting.5 Not only does POCUS work well for this purpose, it is one of the easiest ultrasound exams to learn, perform, and interpret. It can be done with any of the three commonly available probe types (linear, curvilinear, or phased array). The probe is placed in the chest oriented vertically between ribs. The presence of B-lines in multiple lung fields indicates pulmonary edema6,7 and the number of B-lines correlates with severity.8 This is a simple exam prehospital providers can use to more accurately guide treatment of patients with dyspnea.
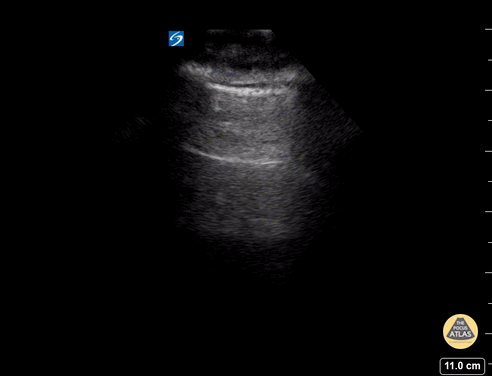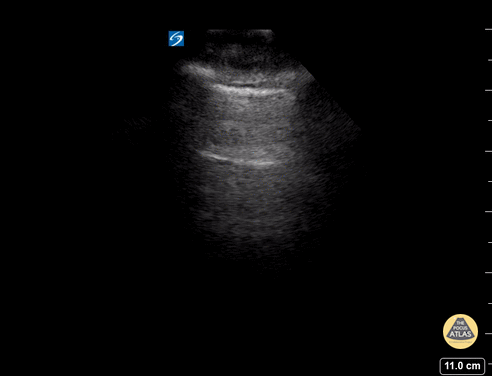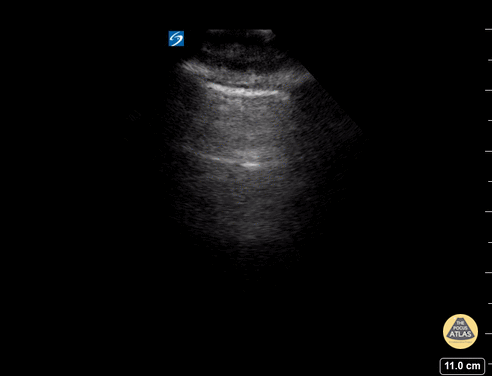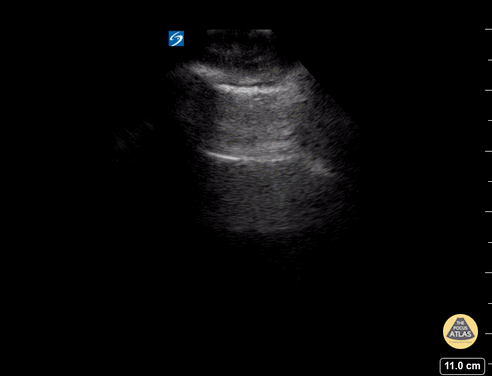
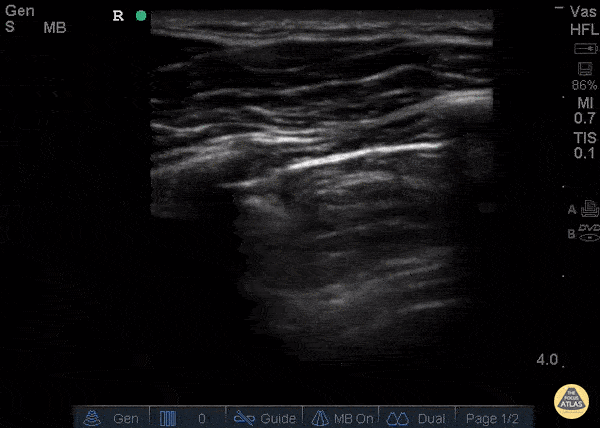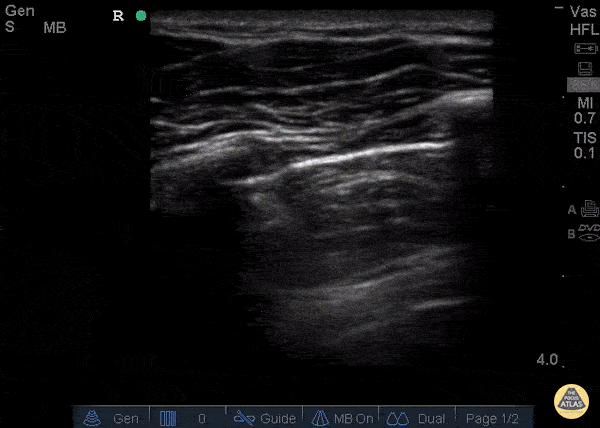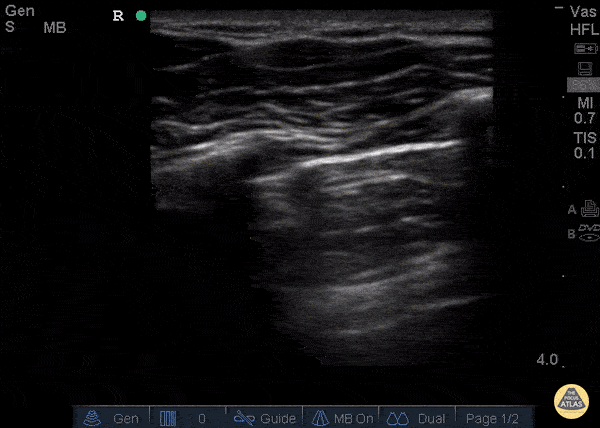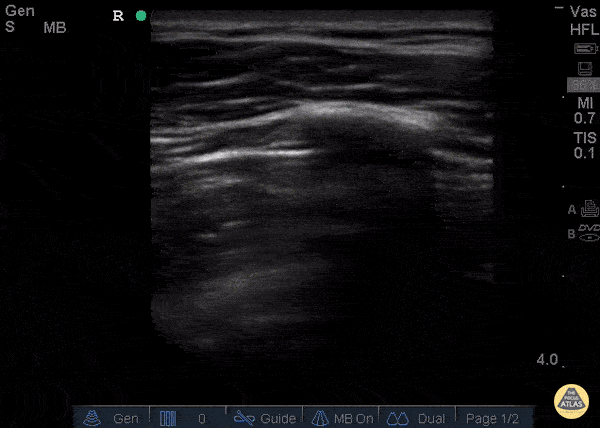
GIF Image: A lines
Caption: Normal appearing lung with horizontal A lines and no sign of B lines
GIF Image: B lines
Caption: B-lines visible coming from pleural line down. They appear to dance around with respiration.
GIF Image: Severe B Lines
Caption: Many B-lines indicating severe pulmonary edema in the right clinical context.
Lung Ultrasound to Determine Pneumothorax
Now, consider a different scenario. Your patient is a 32-year-old male ejected from a motor vehicle after a crash. He is lying in the road in obvious respiratory distress. He is tachycardia, hypoxemic, and has diminished lung sounds bilaterally. What is the next goal of treatment?
It is extremely difficult to diagnose pneumothorax in the prehospital setting. This is because lung sounds are not always reliable as referred lung sounds can be deceiving. Additionally, patients may have respiratory distress and bilaterally diminished lung sounds for other common reasons including hypovolemic shock and pulmonary contusion. Needle decompression and finger thoracostomy are infrequently performed, sometimes daunting procedures, so having some reassurance that a pneumothorax is truly present makes the decision easier.
POCUS has been shown to be extremely accurate for detecting pneumothorax, even more so than chest x-ray.9 It is performed using the linear probe placed vertically between the ribs. In a normally functioning lung, the pleural line can be seen moving back and forth across the screen with respiration. In pneumothorax, there is an absence of lung sliding which can be seen obviously. M-mode can also be used to assess for lung sliding. With normal lung sliding M-mode will show a classic “seashore sign” while absent lung sliding will have the classic “barcode sign.” Absent lung sliding can be seen in other conditions including acute respiratory distress syndrome (ARDS), pulmonary fibrosis, large consolidations, pleural adhesions, atelectasis, right mainstem intubation, and phrenic nerve paralysis,10 but these are rare and not as likely in an acutely injured patient.
GIF Image: Normal lung slide
Caption: The pleural line appears bright white running between the ribs and moves with respiration.
GIF Image: No lung slide
Caption: In this example the pleural line does not move with respiration.
Image: M-mode
Caption: On the left there is the “seashore sign” indicating lung slide. On the right is the “barcode sign” indicating no lung slide and possible pneumothorax.
These two applications of lung ultrasound can dramatically impact the care given in the prehospital setting. It provides definitive information about the underlying pathology that cannot otherwise be obtained. There are many incredibly important out-of-hospital applications of POCUS. But, if there is one prehospital application of POCUS every advanced prehospital provider should have available, it is lung ultrasound.
All ultrasound clips are freely available and open access from The POCUS Atlas
References
- Gheorghiade M, Follath F, Ponikowski P, et al. Assessing and grading congestion in acute heart failure: a scientific statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail. 2010;12(5):423-433. doi:10.1093/eurjhf/hfq045
- Martindale JL, Noble VE, Liteplo A. Diagnosing pulmonary edema. Eur J Emerg Med. 2013;20(5):356-360. doi:10.1097/MEJ.0b013e32835c2b88
- Volpicelli G, Cardinale L, Garofalo G, Veltri A. Usefulness of lung ultrasound in the bedside distinction between pulmonary edema and exacerbation of COPD. Emerg Radiol. 2008;15(3):145-151. doi:10.1007/s10140-008-0701-x
- Ang S-H, Andrus P. Lung ultrasound in the management of acute decompensated heart failure. Curr Cardiol Rev. 2012;8(2):123-136. doi:10.2174/157340312801784907
- Zanatta M, Benato P, De Battisti S, Pirozzi C, Ippolito R, Cianci V. Pre-hospital lung ultrasound for cardiac heart failure and COPD: is it worthwhile? Crit Ultrasound J. 2018;10(1):22. doi:10.1186/s13089-018-0104-5
- Wang Y, Shen Z, Lu X, Zhen Y, Li H. Sensitivity and specificity of ultrasound for the diagnosis of acute pulmonary edema: a systematic review and meta-analysis. Med Ultrason. 2018;1(1):32. doi:10.11152/mu-1223
- Theerawit P, Touman N, Sutherasan Y, Kiatboonsri S. Transthoracic ultrasound assessment of B-lines for identifying the increment of extravascular lung water in shock patients requiring fluid resuscitation. Indian J Crit Care Med. 2014;18(4):195-199. doi:10.4103/0972-5229.130569
- Cortellaro F, Ceriani E, Spinelli M, et al. Lung ultrasound for monitoring cardiogenic pulmonary edema. Intern Emerg Med. 2017;12(7):1011-1017. doi:10.1007/s11739-016-1510-y
- Zhang M, Liu Z-H, Yang J-X, et al. Rapid detection of pneumothorax by ultrasonography in patients with multiple trauma. Crit Care. 2006;10(4):R112. doi:10.1186/cc5004
- Husain LF, Hagopian L, Wayman D, Baker WE, Carmody KA. Sonographic diagnosis of pneumothorax. J Emerg Trauma Shock. 2012;5(1):76-81. doi:10.4103/0974-2700.93116
- LITFL Ultrasound Library. https://litfl.com/ultrasound-library/
Administrative Corner
The Q’s & A’s of Ultrasound Quality Assurance
Alexis Salerno, MD, Allison Zanaboni, MD and Michael Gottlieb, MD
This month we will be discussing quality assurance (QA). QA is importance for any department that uses clinical ultrasound. QA helps to ensure there is a standard for quality of your ultrasound exams and helps to educate providers in your department. As an ultrasound director, it is your chance to give feedback to providers on both technical and clinical grounds. There are many ways that quality assurance can be conducted in your department. This month we have asked Dr. Allison Zanaboni and Dr. Michael Gottlieb their take on a few QA topics.
Question 1: What is your current system or QA?
AZ: Just this academic year, we switched from using an old system for QA called SonoSite Workflow Solutions to Qpath E. Residents are asked to document a worksheet in Qpath E for each study they complete in the ED, and then these are reviewed on a rolling basis by the ultrasound faculty. Qpath E allows us to give credit for studies that meet credentialing criteria, but also gives us the ability to comment on the obtained images and leave feedback and then notifies the residents that their studies have been reviewed. This system allows us to not only easily tract scan totals for credentialing purposes but also allows us to give formative feedback to help improve competency regarding point of care ultrasound.
MG: We use QPath E (Cloud Version) for our quality assurance program. Images are sent directly upon exiting the ultrasound examination and residents log in to complete the QA forms.
Question 2: What percentage of studies (billable, credentialing) do you QA?
AZ: Our goal has been to QA 100% of studies. Certain studies that were not completed or when no adequate images were able to be obtained may not be submitted for QA, and those studies are also not billed for.
MG: We QA every exam that is completed. Our ultrasound division team alternates performing QA on a weekly basis. Whoever is on QA for a given week checks the QA at least once per day.
Question 3: How does your institution differentiate from billable/credentialed studies?
AZ: Most of our faculty are credentialed and can therefore supervise resident studies. These studies, when performed to obtain clinical information, are billed for as long as a procedure note is entered in the EMR. When residents are working with non-credentialed faculty or choose to perform a study for purely educational purposes, they can still submit a worksheet through Qpath and, if adequate for credentialing, receive credit towards their US scan totals.
Question 4: Are there any specific things you are looking for while performing QA?
AZ: My main objectives when reviewing scans is to:
I: Make sure there are no missed findings and, if they are present, ensure the patient is contacted, notified of the findings, and appropriate follow up is arranged
II: Identify areas for improvement in the scan and provide this feedback to the performing resident
III: I also double check to make sure that studies that were used for clinical decision making had an appropriate note placed in the chart for billing purposes as well
MG: When performing QA, I usually begin by assessing image quality. Our QA form includes both an image quality rating, as well as a comments area where we provide feedback for the learner. In the comments area, I make notes of what was done well and areas for improvement with regard to image acquisition. Then, I perform my own blinded interpretation based on the images. I compare this with the interpretation of the person who performed the scan. This allows me to assess the quality of interpretation and provide feedback on that component, as well. Finally, I look at the confirmatory test (when available) or final diagnosis to see how the images and initial interpretation correspond to the final diagnosis.
Question 5: For studies with errors, what is your method of communication to the providers?
AZ: The operators who performed the study are notified via Qpath. If the fining is time sensitive, the resident administrative process that is in place will contact that patient to notify them of the finding. For incidental findings that can wait, the resident involved in the patient’s care or the resident who performed the study will be notified both through Qpath and email to notify the patient.
MG: For studies with quality or interpretation areas of no clinical significance, we email the person who performed the scan. When there is a clinically significant finding, we reach out to both the person who performed the scan and the supervising physician (if applicable), who will subsequently contact the patient to discuss the findings.
Question 6: What about providers who continuously have errors in studies?
AZ: This fortunately has not been too much of an issue. For residents who have self-identified as needing additional US education, we offer an elective US rotation as well as have an open door policy for our weekly educational QA sessions and these residents are encouraged to join to learn from both their scans and other scans performed in the department.
MG: When providers have consistent errors, I believe it is important to discuss it with them and work together to improve the specific area. We have 10 ultrasound workshops throughout the year, as well as four weeks of dedicated ultrasound rotation time where residents work directly with ultrasound fellowship-trained faculty members who are present for every single day of the rotation. This allows us to identify and work together to address any consistent errors early in the course.
Question 7: How often do you find incidental findings on QA, what is the process for this?
AZ: Incidental findings not needing emergent follow up are directed back to the original providers for clinical correlation and instructions to follow up with the patient to make them aware of the findings and appropriate follow up.
MG: We have found relatively few incidental findings of clinically significant on our QA. The most common findings are typically simple renal cysts. For any clinically significant findings, we contact the sonographer and the supervising physician (if applicable) and ensure the patient is informed of the findings. This includes both contacting the patient, as well as documenting in the patient medical record.
Ultrasound Saves
Point of Care Ultrasound for Assessment of Soft Tissues
Shawn Sethi, DO
Case Presentation
A 62-year-old male with history of hepatitis C presents to the emergency department for pain, swelling and redness to his left hand for the last 8 days. This was the patient’s third visit to the ED for the same complaint. After his first visit he was started on cephalexin for suspected cellulitis and finished the course of antibiotics 2 days ago. He denies other complaints including fevers, chills, nausea, vomiting, purulent drainage, or bleeding.
Vital signs are remarkable for mild tachycardia with no fever. On examination the patient has moderate erythema over the dorsum of his left hand extending proximally over the wrist and distal forearm with associated soft tissue swelling and pain to palpation. There are no open wounds, purulence, induration, or fluctuance noted. The patient has a 2+ radial pulse, normal light touch sensation and no pain with passive or active range of motion at the wrist or fingers.
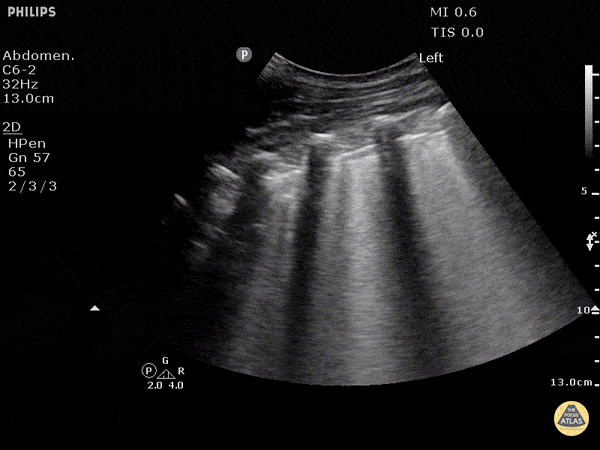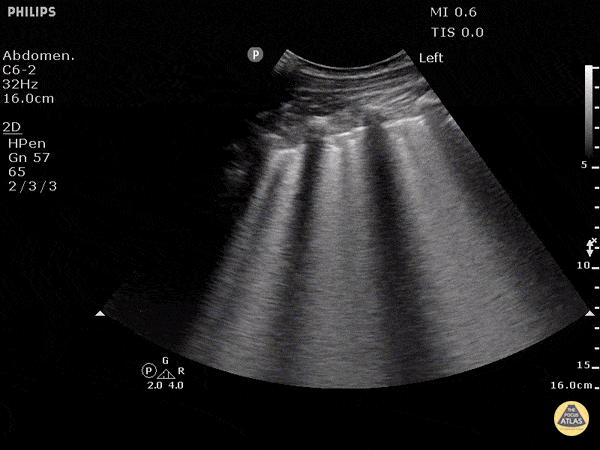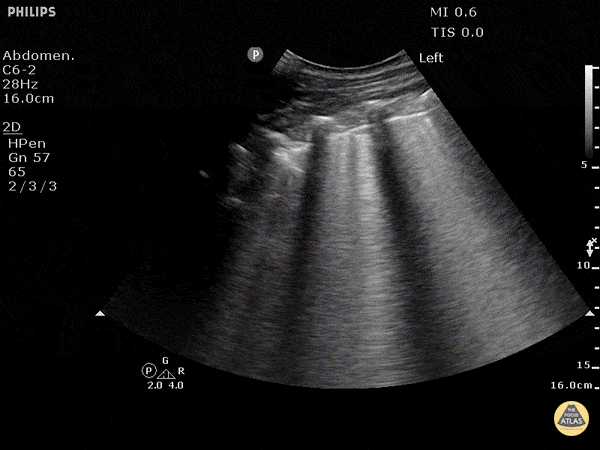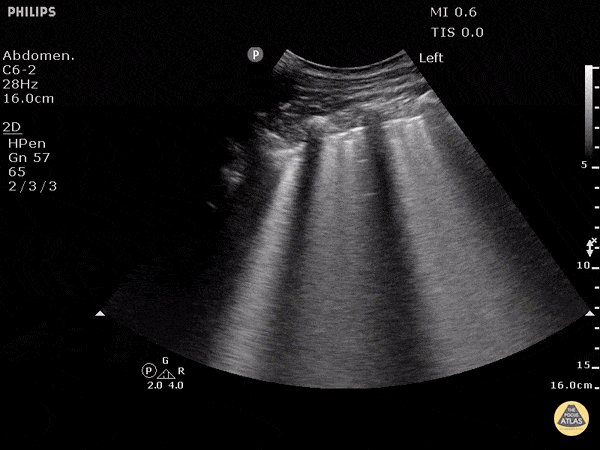
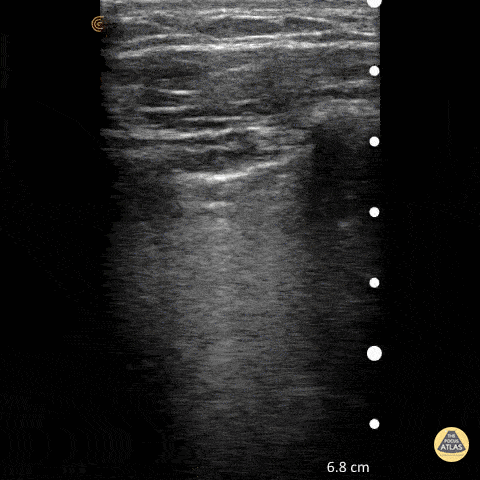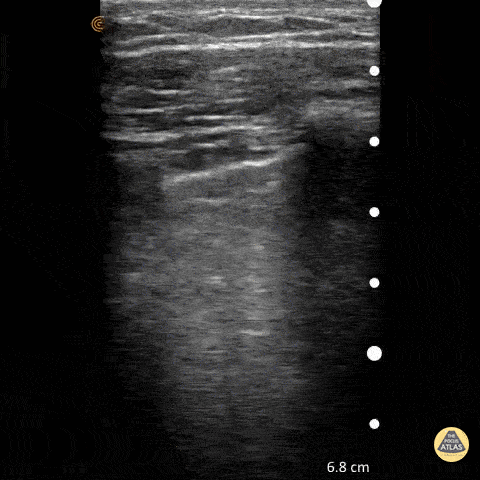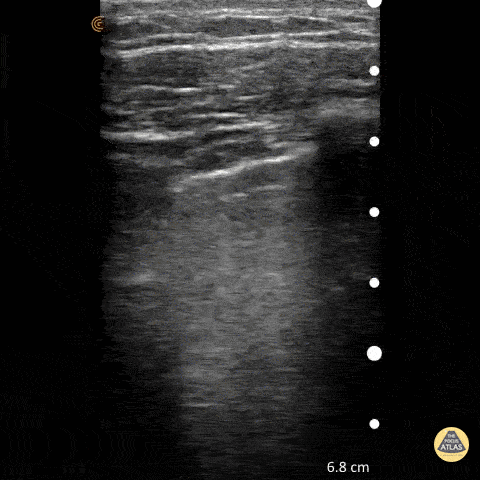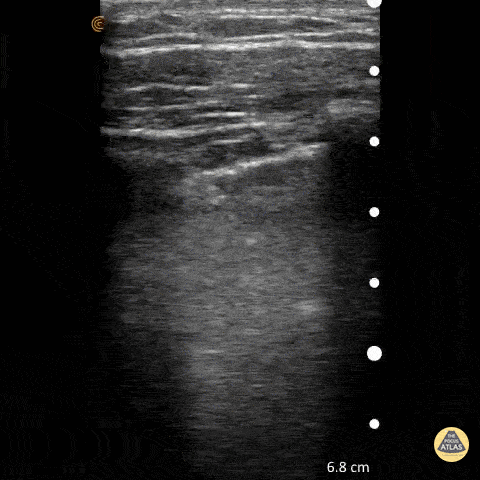
Point of care ultrasound (POCUS) was used to further evaluate the progression of the patient’s symptoms. What’s your diagnosis based on the image below?
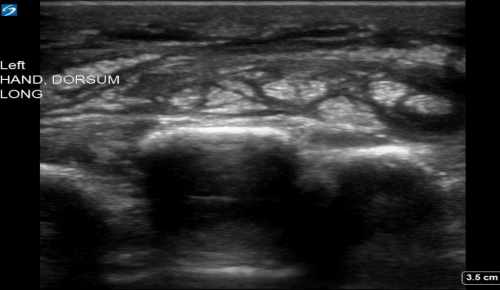
Figure 1: Transverse view of left hand using linear probe
Answer
Cellulitis
Discussion
Cellulitis is a common emergency department diagnosis, but it can be surprisingly difficult to diagnose abscess formation on physical exam. A 2016 systemic review compared physical examination to POCUS plus exam in differentiating cellulitis from abscess. The authors found that POCUS plus physical examination out-performed examination alone. These findings may be even more significant for pediatric populations (which were included in this review) as POCUS could avoid unnecessary and risky procedural sedations.1 In addition, a 2019 trial by Gaspari et al. analyzed patients with soft tissue abscesses and compared failure rates after treatment with incision and drainage with or without assistance of ultrasonography. The authors found that patients who underwent POCUS to assist with confirmation and drainage of the abscess had lower treatment failure and improved clinical cure rates.2
Musculoskeletal ultrasound is performed using a linear (high frequency) probe. The probe is used to scan the area of interest in both the short and long axis. Extremely superficial structures, such as the flexor tendons of the hand, may be difficult to visualize. Using a water bath can allow the sonographer to increase the stand-off distance of the probe and improve image quality. When encountering an anechoic structure of unsure etiology, visualizing the structure in both planes and using color or power doppler for further interrogation can assist with the differential of vascular and cystic structures.
The characteristic ultrasonographic finding in cellulitis is known as “cobblestoning.” As cellulitis progresses more edema will fill the spaces between hyperechoic fat lobules. Cobblestoning is caused by fluid tracking through the subcutaneous tissues or along fascial planes. In contrast, abscesses will appear as a single large fluid collection with heterogeneous echotexture due to the debris with them. In this case, ultrasound confirmed the patient’s diagnosis of cellulitis leading to IV antibiotics and admission for failure of outpatient treatment.
POCUS for soft tissues can be used for not only diagnostic purposes and avoiding potential unnecessary procedures and imaging, but also has therapeutic value in improving a very common bedside procedure.
References
- Subramaniam S, Bober J, Chao J, et al. Point-of-care ultrasound for diagnosis of abscess in skin and soft tissue infections. Academic Emerg Med. 2016; 23: 1298-1306. https://doi.org/10.1111/acem.13049
- Gaspari R, Sanseverino A, Gleeson T. Abscess incision and drainage with or without ultrasonography: a randomized controlled to. Annals Emery Me. 2019; 73: 1-7. https://doi.org/10.1016/j.annemergmed.2018.05.014
From Journal to Practice
Cardiac Standstill: How Do You Know When It’s Time to Stop?
Jared L. Cohen, MD MA and Melissa Myers, MD FAAEM
You are working overnight in a community ED and are called up to the internal medicine floor to evaluate a patient in extremis. When you arrive, you see a 90-year-old female in cardiac arrest. The initial rhythm is pulseless electrical activity. You run the code for 20 minutes and are considering ceasing resuscitative efforts. The nurses working with you are upset at the thought of stopping and say she was admitted for a urinary tract infection. You are considering using cardiac standstill as a prognostic indicator but aren’t sure what to look for or if there is strong evidence.
Cardiac standstill has been used as a predictor for survival after cardiac arrest for many years. A 2016 study by Gaspari et al. evaluated patient survival after cardiac standstill. 793 patients were enrolled in multiple hospitals in the United States and Canada.1 In this cohort, the presence of cardiac activity was the factor most strongly associated with survival to hospital admission. Of the 530 patients without cardiac activity, only three survived to hospital discharge.1 This outcome suggests that it is reasonable to incorporate the absence of cardiac activity as an indicator to stop resuscitative efforts.
An important question to consider is how the research defines cardiac activity. Unfortunately, there is not a clear, consistent definition for what qualifies as standstill. This stems from the fact that standstill, on the surface, seems like a topic that is self-explanatory. If the heart is not having meaningful movement, it is at standstill. This simple definition breaks down when applied clinically, and recent research shows a surprising lack of agreement amongst physicians in evaluating possible cardiac standstill.
Often times, evaluating the excursion of the anterior mitral valve leaflet is the method of choice to evaluate an ejection fraction (EF). It is a visual interpretation based upon the mitral valve leaflet excursion and distance from the ventricular septum.2 However, this is ultimately a surrogate marker for the amount of blood ejected from the ventricle. Direct measurement of the ejection fraction is based on the change in chamber size from diastole compared to systole, but this method is more complex and often avoided in emergency medicine education.
In a 2018 study by Hu et al., 127 physicians were asked to decide if a 15 second ultrasound clip showed cardiac standstill. Interrater agreement for determining this critical event was surprisingly low (a = 0.47).3 While this, by statistical standards, demonstrates a moderate level of agreement, previous research essentially assumed that reliability between physicians would be so high that they did not even need to include a specific definition for cardiac standstill. For example, in the Gaspari et al. study previously discussed, they note “no pre-existing standard for defining cardiac activity on ultrasound” so cardiac activity in their study “was defined a priori as any visible movement.”1
In the Hu et al. article, five examples of standstill were largely responsible for the worrisome interrater reliability.3 In these examples, valve flutter was present and some had left ventricular chamber movement while others did not. Our teaching model with the mitral valve leaflet as a surrogate for ejection fraction may lead us astray in evaluating standstill. Valve fluttering in PEA is not the same as a change in ventricular chamber size. Looking at a fluttering valve can easily lead one to interpret that there is forward blood flow when the ejection fraction is in fact zero. One’s method for determining the ejection fraction is often the method used to evaluate standstill. For example, if you are used to focusing on the mitral valve for your assessment of global function, that is likely where you look when evaluating a patient in PEA.
A 2018 meta-analysis performed by Wu et al evaluated the ability of to predict return of spontaneous circulation (ROSC) in patients with PEA. They included eleven studies from 2001 to 2016 encompassing 777 subjects. 4 The patients were sorted into two groups, ones with cardiac standstill called “true-PEA” and ones with cardiac activity called “Psuedo-PEA.” In their standstill group, 42 of 343 patients obtained ROSC while 188 of 434 patients with cardiac activity on ultrasound obtained ROSC. Of the eleven studies included, the definition for cardiac activity varied. Four studies defined cardiac activity as “any cardiac motion,” three defined it as “ventricular movement,” one as “any valve motion,” and three did not specify a definition. This variance in definitions for cardiac activity amongst the research limits our interpretation of this data.
Given that physicians rely on identification of cardiac standstill to cease resuscitative efforts, there is obviously a worry over false positive and false negative interpretations. More importantly, it is difficult to interpret the majority of prior research on this topic since there is not a consistent definition. If your interpretation of standstill is different from that of the physicians in the research, the prior results are not clinically helpful.
Implementation of a unified definition of cardiac standstill would likely increase the interrater agreement amongst physicians. Returning to an ejection fraction definition revolving around the change in chamber size, at least in the evaluation of an arresting patient, may enhance our specificity and ultimately our external validity of each study. Valve movement causes confusion in this unique circumstance. The use of ultrasound for cardiac standstill could enable emergency physicians to appropriately direct or cease resuscitative efforts, but standardization is needed to reliably research and provide meaningful outcome metrics in the setting of the arresting patient.
References
- Gaspari R, Weekes A, Adhikari S, et al. Emergency department point-of-care ultrasound in out-of-hospital and in-ED cardiac arrest. Resuscitation. 2016. doi:10.1016/j.resuscitation.2016.09.018
- Emergency Ultrasound. O.J. Ma, J.R. Mateer, editors. McGraw-Hill Ryerson Ltd. $170. 435 pp. ISBN 0-07-137417-5. Can J Emerg Med. 2003. doi:10.1017/S1481803500015955
- Hu K, Gupta N, Teran F, Andrus P. Variable interpretation of cardiac standstill among physician sonographers. Acad Emerg Med. 2016. doi:http://dx.doi.org/10.1111/acem.12974
- Wu C, Zheng Z, Jiang L, et al. The predictive value of bedside ultrasound to restore spontaneous circulation in patients with pulseless electrical activity: A systematic review and meta-analysis. PLoS One. 2018. doi:10.1371/journal.pone.0191636
Letter to the Editors
What stood out to you from this issue of the POCUS Report? Have a question, idea, or opinion? Alexis Salerno, MD and Melissa Myers, MD FAAEM welcome your comments and suggestions. Submit a letter to the editor and continue the conversation at https://form.jotform.com/90834609522156.
Get Involved
We encourage original submissions to this e-newsletter and to our recurring section in Common Sense. Please contact us with any questions at EUS@aaem.org.